The biological approach for sternal replacement: sternochondral allograft transplantation
Introduction
Chest wall resection and reconstruction is a challenge for thoracic surgeons particularly when the sternum is involved. Sternal resection could be necessary for different pathologies such as primary or metastatic tumors, infection related or unrelated to previous sternotomy, radiation-induced necrosis, and trauma (1-3). Various materials and techniques have been used for sternal replacement, and even today, a gold standard procedure has still not been defined (4). In 2010, our group reported the first case of allograft sternochondral replacement after resection of a large sternal chondrosarcoma (5). Herein, we report our definitive experience with allograft sternal reconstruction of the chest wall and comprehensively review the literature from around the world reporting on the use of this technique.
Methods
Between February 2008 and October 2018, 14 patients underwent sternectomy followed by anterior chest wall reconstruction using cadaveric cryopreserved sternal allograft. The present study obtained ethics approval from the institutional review board of Padua University Hospital (PD00-19-03CT). Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images. Patients’ characteristics are reported in Table 1. Patients consisted of 9 females and 5 males with a median age of 61 years (range: 35–78 years). Indications and related case numbers for sternal resection and reconstruction were as follows: single-site metastatic disease in 4 patients, primary chondrosarcoma in 6 cases, sternal dehiscence after cardiac surgery in 1 case, direct sternal involvement from thymic carcinoma in 1 case, soft tissue sarcoma in 1 case, and plasmacytoma in 1 case. Chest X-ray, computed tomography (CT), and positron emission tomography (PET) were respectively used for the precise definition of the tumor, the evaluation of sternal involvement, or the assessment of sternal dehiscence (Figure 1). Chest magnetic resonance imaging (MRI) was performed when a suspicion of mediastinal or thoracic outlet involvement arose. The median major diameter of the tumor was 6 cm (range: 3–21 cm).
Full table

Every patient was routinely evaluated with cardiopulmonary tests. In 11 patients affected by neoplasm, the diagnosis was obtained by performing an open biopsy or preoperative CT or ultrasound-guided needle biopsy.
The site of sternal involvement was the body of the sternum in 8 patients; the manubrium and body of the sternum 5 patients; and the manubrium, clavicles, and upper body in 1 patient.
Three patients received preoperative induction therapy, 2 received chemotherapy, and 1 received chemo-radiotherapy.
Allograft procurement and conservation
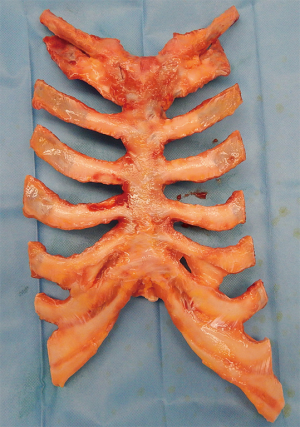
Sternal allografts were requested from the tissue bank who were provided with all the anthropometric measurement data of the recipients. The sternochondral block was harvested from a multiple-tissue donor in a completely aseptic technique following the rules of the Italian legislation regarding organ donation. The allograft was then treated by immersion in a sterile saline solution added with antibiotics for 72 hours at 4 °C. The subsequent step consisted of irradiation and storage at −80 °C after packaging. This process guarantees sterility of the allograft and the absence of immunogenic capacity. Moreover, this treatment destroys the major histocompatibility complex, and thus ABO or human leukocyte antigen matching is not required for the same reason that no immunosuppressive therapy is necessary after a graft implant. An x-ray picture of the sternochondral block was taken by the tissue bank operator and sent to us for final size matching between the donor sternum and the recipient’s thoracic cage. The day before surgery the allograft underwent defrosting up to 4 to 6 °C for 12 hours. The day of the operation in the surgical theater, the graft was unpacked and immersed in sterile 0.9% sodium chloride solution to complete thawing just before implantation (for detailed information: www.bancatessuti-treviso.org) (Figure 2).
Surgical technique
All the operations were performed under general anesthesia. Double-lumen intubation was used in cases of planned associated lung resection; otherwise, the operation was performed with single-lumen intubation.
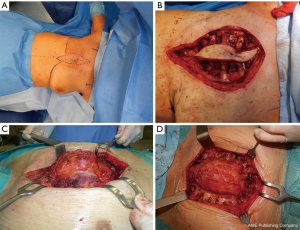
The surgical technique of allograft transplantation has been previously described in detail (5,6). Briefly, the patient was placed in supine decubitus position with the neck hyperextended placing a roll behind the shoulders (Figure 3A). A midline skin incision was performed in cases of non-neoplastic patients or when the sternal tumor was confined between the cortical layer of the sternum. A different kind of incision was performed in cases of larger tumors and when subcutaneous or even musculocutaneous tissue infiltration was documented, by leaving at least 3 cm from the resection margins (Figure 3B,C,D). In most simple cases, both pectoral muscles were prepared to be used as a flap to cover the anterior chest wall reconstruction. In cases of more extensive demolition, plastic surgeons were involved to prepare different muscles or myocutaneous-free or pedunculated flaps for more complex reconstruction (Figure 4). Depending on the type of pathology, a full thickness, partial, or complete sternectomy en bloc with costal cartilage was performed. In case of locally advanced tumors, it was sometimes necessary to perform enlarged resections to other mediastinal structures or lungs parenchyma.
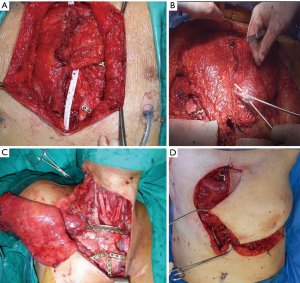
In all cases, it is crucial to extensively dissect the intercostal spaces as far as the anterior axillary line to be sure to expose the anterior costal arches in which the cortex of the bone is strong enough to house the screws and titanium bars during the reconstructive phase of the procedure. The allograft is tailored to fit perfectly within the chest wall defect using an oscillating saw. Care must be taken to avoid the overlapping of the sternal edges after partial sternectomy or misalignment of the costal segments. It is mandatory to avoid excessive reduction of the allograft leaving excessive space between the thoracic cage of the recipient and the implanted new sternum. Indeed, an unstable reconstruction with chronic movement of the implanted allograft could lead to chronic chest pain, fractures of the titanium bars, or dislocation of the screws with failure of the entire reconstruction. After the tailoring, the titanium bars (Synthes, Solothurn, Switzerland) that we used to fix the allograft to the recipient chest wall were modelled to the best fitting shape on the basis of the previously identified anchor points on the patient’s ribs and sternal allograft (Figure 5). The bars were fixed with screws on well-represented bone tissue avoiding the costal cartilages; otherwise, screw dislodgment is unavoidable. For every titanium bar, we used at least 2 screws for each side, with the size of the screw being related to the bone thickness which was evaluated with dedicated tools (Synthes, Solothurn, Switzerland). In cases of partial sternectomy, an H-shaped titanium plate was used to fix the sternal allograft to the residual sternum of the recipient. We also placed at least 2 screws for each side of the plate, and, in cases of very thin and fragile sternums, alternatively, two straight titanium bars can be used as a bridge between the allograft and the recipient sternum using in this case at least 3 screws for each side. In cases of manubrium reconstruction, different techniques were used to recreate the sternoclavicular joint, such as high-tension polyethylene sutures, titanium bars, or intramedullary nails. The fixation of the clavicle to the “new” sternum presented a particularly challenging situation. The high-tension sutures were passed through the clavicle head and the manubrium sterni after making small holes using a drill. Generally, we used two high tension sutures for each clavicle. When the clavicle head is resected en bloc with the manubrium, we prefer to use intramedullary nails, one for each clavicle, that are fixed to the “new” manubrium using bi-cortical screws. To achieve better stability of the sternoclavicular joint, it is very important to avoid cutting the ligament between the clavicle and the first rib whenever possible. In all cases, the implanted allograft should be covered with vital tissue, usually a pectoralis muscle flap.
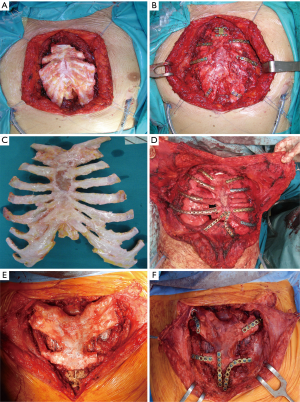
Results
Seven patients underwent partial (lower) sternectomy with preservation of the manubrium, 5 patients received a partial (upper) sternectomy involving the manubrium, clavicles (in one patient) and part of the body, and 2 patients underwent total sternectomy. Resection was extended to the lung (wedge resection) in 1 patient, and to the superior vena cava system in 1 patient. A muscle flap of the pectoralis major was used to cover the graft in 9 patients, and in 1 case, a latissimus dorsi musculocutaneous flap was used after en bloc resection of the sternum and right pectoralis major muscle. One patient had an R2 resection (thymic carcinoma) at the level of aortic arch, and 1 patient had an R1 resection at the level of the clavicle margin (plasmacytoma). All patients were weaned from mechanical ventilation in the operating theater. The mean intensive care unit stay was 1.1 days (range 0–16 days). One patient died in the perioperative period due to a pulmonary embolism on the 8th post-operative day. Minor morbidity in the post-operative period were atrial fibrillation in 1 patient, pleural effusion requiring drainage in 1 patient, and pulmonary atelectasis with necessity of bronchial toilet in 1 patient. As a major morbidity, 1 patient had prolonged fever due to a systemic Candida infection, and 1 patient required surgical revision without redo surgery on the sterno-chondral allograft because of bleeding from the latissimus dorsi muscle flap. The median length of hospitalization was 11 days (range: 6–31 days).
Follow-up
All patients were followed up by clinical visit with a dosage of tumor marker and total body CT scan every six months, to exclude recurrence of tumor or infection, and to check the stability and the correct localization of the graft. Then, for neoplastic diseases, a CT scan was performed every 4 to 6 months for the first 2 years after surgery, then yearly. After a median follow-up of 54 months (range: 19–137 months), neither infection nor rejection of the graft occurred. Because of partial dislocation, 1 patient underwent surgery to remove a clavicular screw 4 months after the operation. Eight patients are alive, and 6 are dead (4 patients died of systemic recurrence of the primary disease, 1 patient of a cerebral vascular accident, and 1 patient died of septic state). No respiratory impairment or flail chest occurred in any patients. After surgery, 3 patients underwent adjuvant therapy, 2 patients who were affected by metastases of breast cancer received chemotherapy, and the patient with plasmacytoma received radiotherapy.
Discussion
Since the first chest wall reconstruction described by Tesini in 1906 (7), this surgery has evolved with the introduction of new materials and different techniques (4). Despite the fact that a gold standard procedure for chest reconstruction is still far from being defined, a general agreement about the primary goals of chest wall reconstruction has been widely acknowledged in the literature (1-4). A correct and effective reconstruction should eliminate any dead space, preserve the respiratory movements and mechanics so as to even improve the restoration of chest wall rigidity, protect the intrathoracic organs, and minimize chest cage deformity. In oncologic patients, the reconstruction should not exclude the possibility of receiving adjuvant radiotherapy or interfering with radiological follow-up (8,9). Nowadays, different materials are available for chest wall repair and they can be classified mainly into synthetic, rigid, or flexible types; biologic, autologous, or heterologous types; and rigid tissue or soft tissue types.
Every material has its advantages and disadvantages, and none of them have been proven to be clearly superior. The decision to use one or the combination of several different materials depends on the preoperative and intraoperative evaluation and prognostic factors. The type of pathology, the location and the size of the defect, the risk of infection, the materials that are available, and the surgeon’s experience or preferences are the main factors (4).
In case of anterior chest wall resection in particular, if the sternum is involved, the reconstruction requires rigid materials and very frequently the combination of those with viable tissue coverage such as using muscle flap, omental flap etc. (1-4).
Synthetic rigid materials have the disadvantages of excessive rigidity, the risk of erosion of mediastinal structures, the risk of infection, rupture due to insufficient strength and flexibility, the risk of migration, frequent interference with following imaging studies, and the inability to be incorporated in to the host tissue (5,6,8).
In the group of biological rigid materials, bone autografts are extensively used with different anatomical options like the iliac crest, rib segments, etc. (4). This type of biological material has optimal biomechanical performance, and is fully compatible (5,6). Obviously, the amount of bone tissue that could be harvested from the patient is limited, and in the case of large defects, additional aesthetic and functional insult with complex surgical harvesting in other body areas further compromises the patient’s condition and quality of life. In 1996, Puma et al. (9) reported their preclinical and clinical experience using bone heterograft as a substitute for the sternum. They convincingly demonstrated that a heterograft offers the same advantage of the autograft but limits the patient’s surgical trauma significantly. Moreover, heterograft bone is readily available, and so the surgeon can preoperatively choose different bone segments of various shape and sizes. The surgical procedure is faster and easier than with autologous tissue, the cortical graft has no tendency to be reabsorbed during follow-up, and, theoretically, the spongy tissue can act as a scaffold for osteoprogenitor cell migration, neovascularization, and new bone formation as in case of an autograft. In the literature, different reports published throughout the years attest to good results obtained from the use of a bone heterograft for sternal reconstruction. Different bone segments like the iliac crest, ribs, calva bone, fibula segment, etc. have been used (4,9-11). If in thoracic surgery the experience of heterograft use is still limited, in other specialties like orthopedics or maxillofacial surgery, it is an already established reality with long-term follow-up with excellent results being reported (12,13). Chest wall reconstruction with bone autografts or heterografts always requires a combination with other materials to fix the implanted bone to the patient’s chest cage. Generally the most used material is titanium in varying shapes of bars, plates, meshes and screws (4-6).
Titanium is highly biocompatible with low-density bone, resistant to corrosion, ductile, diamagnetic, and compatible with MRI. Titanium is as resistant as steel but 40% lighter; it weighs 60% more than aluminum but has double the strength. The plethora of these positive features justifies the extensive use of this material in surgery (4). Moreover, among the synthetic materials used for the reconstruction of the chest wall, titanium is the most resistant to bacterial colonization as reported in animal and human studies. The specific surface/interface design of titanium is known to make the surface of biomaterial less interactive (14-16).
At the end of implantation, a new sternum fixed with titanium must be covered with viable tissue, which is generally the pectoralis muscles flap. When this is not suitable, other muscles, either myocutaneous pedunculated, or free flaps, have to be used. The muscle flap guarantees good blood supply, contributes to chest wall stability, reduces the risk of infection, and improves cosmetic results (5,6).
In 2010 (5), our group published the first case of sternal reconstruction using a sternochondral allograft after sternectomy for chondrosarcoma. In 2012, Stella et al. (17) published a second case of sternal reconstruction after sternectomy for ovarian carcinoma metastasis. In both cases, the result was excellent in terms of biomechanical support to ventilation and cosmetic result; both patients were still alive after 10 and 8 years without complications related to the surgical technique and were free from cancer recurrence. This new technique using a sternal allograft is highly reproducible mainly because when a sternum is used to replace a sternum, it guarantees an excellent matching between the implanted bone and the recipient chest wall defect, thus avoiding the necessity of the complicated tailoring of the other bone segment. During the preoperative work-up, sharing the patient’s chest wall dimensions and diameters of the sternum at the level of the manubrium, body, and the length between the jugular fold and the xifoid process with the tissue bank allows for the possibility to choose the perfect size-matched multi-tissue donor. Future technologies, such as surgical-guided navigation, and the use of CT scanning to print 3D models to simulate the procedure and the entity of the resected bone, could allow us a further improvement in size-matching and reconstruction techniques (18,19).
In the last few years, allograft sternochondral replacement has gained popularity (6,20-24).
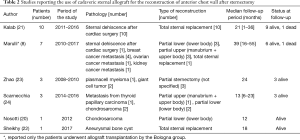
In 2016, Kalab et al. (21) published their experience using an allogenic sternal graft to reconstruct massive post-sternotomy defects after cardiac surgery. They treated 10 cases with excellent results, after a mean follow-up time of 21 months; only 1 patient died 5 months after the operation.
Their experience is particularly noteworthy in that it demonstrates that sternal allograft is very resistant to infection and could be used in cases of sternal dehiscence and mediastinitis with good results. Similar results in post sternotomy dehiscence and infection were achieved by other groups (25). In Table 2, we display a short description of the published cases of sternal transplantation published until today. Excluding the cases treated in our institution, to our knowledge, other 25 patients underwent sternal replacement with cadaveric sternum in different departments. From published data, 23 patients are still alive at the follow-up, and none of the patients have died due to causes related to the chest wall reconstruction. The mean follow-up time of these reported cases is 22.6 months. Allograft implantation was used in 15 patients because of neoplastic disease and in 11 cases because of sternotomy dehiscence.
Full table
In 2017, our group, in collaboration with the Bologna group (8), published the largest series in the literature of 18 patients treated with allograft sternal transplantation for different pathologies. Post-operative results were encouraging and after a median follow-up of 36 months neither infection nor rejection/necrosis of the graft occurred. Reoperation for reconstruction was never necessary, and at this time, 13 patients are still alive and in good clinical condition, 4 patients died for systemic relapse of the neoplastic disease, and none had local relapse.
Thus far, 14 patients have undergone sternal allograft implantation at our institution, and only 2 patients had complications: 1 from the muscle flap requiring surgical revision, and 1 from failure of the sternoclavicular joint repair 4 months after the operation. After a median follow-up time of 54 months, 8 patients are still alive; the causes of death during follow-up were systemic recurrence of the primary neoplasm in 5 patients and a cerebrovascular accident in 1 patient. No patients were subjected to surgical revision of the implant, and during radiological follow-up, the bone grafts did not show any signs of bone reabsorption or necrosis.
Theoretically, bone allografts have the possibility of being integrated into the host skeleton because of three main biological processes: osteoinduction, osteoconduction, and osseointegration, as reported by Albrektsson et al. (26). The first is the process by which osteogenesis is stimulated in a similar fashion as to what is observed in a normal bone healing phase. Osteoconduction means that the bone is able to grow, and this phenomenon is obviously absent in materials with low biocompatibility. However, what is really important is osseointegration, which is the stable anchorage of the implanted sternum achieved by direct bone to implant contact (27). Kalab et al. (11) performed scintigraphy examination during follow-up in 5 patients undergoing sternal allograft transplants, and documented the high healing activity of the grafts and particularly of the crushed spongy bone.
We performed PET with 18-fluoride in 3 patients to evaluate the vitality of the implanted grafts. We found good activity at the level of the junction between the donor and the host bone that could be seen as an initial sign of osseointegration similar to that which Kalab found in his patients (11). Nevertheless, at this time, no one has been able to demonstrate a new cellular habitation of the implanted graft. Perhaps bone biopsy and “new” bone marrow sampling are the only ways to document it, but, to our knowledge, these have not been performed for reasons of ethical consideration. What is certain, however, is that after long-term follow-up of our first patients, there was stability of the implanted bone. Furthermore, neither necrosis nor resorption occurred in our experience, nor were they reported in any of the published series or case reports (6,20-24).
Regarding financial issues, a sternal allograft in our region costs between 800 and 1,000 euros, with the cost of titanium bars and screws being additional. However, this expenditure is far lower than that reported for the new 3D patient-tailored synthetic prosthesis, that, in our opinion could become the true competitor of the sternal allograft transplantation technique (19,28-31).
Conclusions
Sternal allograft transplantation is a safe, reproducible technique that provides excellent functional and cosmetic results. The allograft is biologically well-tolerated, and no complications related to graft necrosis or rejection have been reported in the literature or in our experience thus far, even in long term follow-up. The use of biological materials, particularly the sternum, could become the procedure of choice in the case of complex sternal dehiscence with or without mediastinitis after cardiothoracic surgery by virtue of its resistance to infections. The migration into the allograft of new bone marrow cells and osteoprogenitor cells should be demonstrated with further histopathological and radiological studies. We hope that the good results obtained in our experience and described in the review of other published series may contribute to the further diffusion of this technique as the first choice in cases of anterior chest wall reconstruction. Further studies are necessary to consolidate the early and long-term results. We should also investigate the new frontiers of technology with innovations such as patient-tailored 3D printed new-sternum prostheses and biological synthetic scaffolds for pluripotent cellular colonization compared with bone allograft.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Francesco Puma and Hon Chi Suen) for the Focused Issue “Surgical Management of Chest Wall Tumors” published in Current Challenges in Thoracic Surgery. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: The Focused Issue “Surgical Management of Chest Wall Tumors” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The present study obtained ethics approval from the institutional review board of Padua University Hospital (PD00-19-03CT). Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chapelier AR, Missana MC, Couturaud B, et al. Sternal resection and reconstruction for primary malignat tumors. Ann Thorac Surg 2004;77:1001-6. [Crossref] [PubMed]
- Lequaglie C, Massone PB, Giudice G, et al. Gold standard for sternectomies and plastic reconstructions after resections for primary or secondary sternal neoplasms. Ann Surg Oncol 2002;9:472-9. [Crossref] [PubMed]
- Leuzzi G, Nachira D, Cesario A, et al. Chest wall tumors and prosthetic reconstruction: a comparative analysis on functional outcome. Thorac Cancer 2015;6:247-54. [Crossref] [PubMed]
- Sanna S, Brandolini J, Pardolesi A, et al. Materials and technique in chest wall reconstruction: a review. J Vis Surg 2017;3:95-109. [Crossref] [PubMed]
- Marulli G, Hamad AM, Cogliati E, et al. Allograft sternochondral replacement after resection of large sternal chondrosarcoma. J Thorac Cardiovasc Surg 2010;139:e69-70. [Crossref] [PubMed]
- Dell’Amore A, Cassanelli N, Dolci G, et al. An alternative technique for anterior chest wall reconstruction: the sternal allograft transplantation. Interact Cardiovasc Thorac Surg 2012;15:944-7. [Crossref] [PubMed]
- Tesini I. Sopra il mio nuovo processo di amputazione della mammella. Gazzetta Med Ital 1906;57:141-2.
- Marulli G, Dell’Amore A, Calabrese F, et al. Safety and effectiveness of cadaveric allograft sternochondral replacement after sternectomy: a new tool for the reconstruction of anterior chest wall. Ann Thorac Surg 2017;103:898-905. [Crossref] [PubMed]
- Puma F, Avenia N, Ricci F, et al. Bone heterograft for chest wall reconstruction after sternal resection. Ann Thorac Surg 1996;61:525-9. [Crossref] [PubMed]
- Dell'Amore A, Campisi A, Giunta D, et al. Surgical options to treat massive sternal defect after failed Robicsek procedure. J Thorac Dis 2018;10:E410-5. [Crossref] [PubMed]
- Kaláb M, Karkoska J, Kaminek M, et al. Reconstruction of massive post sternotomy defects with allogenic bone graft: four year results and experience using the method. Interact Cardiovasc Thorac Surg 2016;22:305-13. [Crossref] [PubMed]
- Baldwin P, Li DJ, Auston DA, et al. Autograft, allograft and bone graft substitutes: clinical evidence and indication for use in the setting of orthopaedic trauma surgery. J Orthop Trauma 2019;33:203-13. [PubMed]
- Canzi G, Talamonti G, Mazzoleni F, et al. Homologous banked bone grafts for the reconstruction of large cranial defects in pediatric patients. J Craniofac Surg 2018;29:2038-42. [PubMed]
- Berthet JP, Solovei L, Tiffet O, et al. Chest-wall reconstruction in case of infection of the operative site: is there any interest in titanium rib osteosynthesis? Eur J CardioThorac Surg 2013;44:866-74. [Crossref] [PubMed]
- Arens S, Schlegel U, Printzen G, et al. Influence of materials for fixation implant on local infection. An experimental study of steel versus titanium DCP in rabbits. J Bone Joint Surg Br 1996;78:647-51. [Crossref] [PubMed]
- Harris LG, Richards RG. Staphylococci and implant surfaces: a review. Injury 2006;37:S3-14. [Crossref] [PubMed]
- Stella F, Dell’Amore A, Dolci G, et al. Allogenic sternal transplant after sternectomy for metastasis of ovarian carcinoma. Ann Thorac Surg 2012;93:e71-2. [Crossref] [PubMed]
- Stella F, Dolci G, Dell’Amore A, et al. Three-dimensional surgical simulation-guided navigation in thoracic surgery: a new approach to improve results in chest wall resection and reconstruction for malignant disease. Interact Cardiovasc Thorac Surg 2014;18:7-12. [Crossref] [PubMed]
- George E, Barile M, Tang A, et al. Utility and reproducibility of 3-dimensional printed models in pre-operative planning of complex thoracic tumors. J Surg Oncol 2017;116:407-15. [Crossref] [PubMed]
- Nosotti M, Rosso L, Medogni P, et al. Sternal Reconstruction for unusual chondrosarcoma: innovative technique. J Cardiothorac Surg 2012;7:40-2. [Crossref] [PubMed]
- Kaláb M, Karkoska J, Kaminek M, et al. Transplantation of allogenic bone graft in the therapy of massive post sternotomy defects – 6 years of experience with the method. Rozhl chir 2016;95:399-406. [PubMed]
- Sheikhy K, Dezfouli AA, Beigee FS. Reconstrauction of chest wall by cryopreserved sternal allograft after resection of aneurysmal bone cyst of sternum. Case Rep Surg 2017;2017:9135657. [Crossref] [PubMed]
- Zhao Y, Peng C, Lui Y, et al. Clinical study of allogenic graft reconstruction for sternum tumor. Exp Clin Transplantation 2016;3:353-7.
- Scarnecchia E, Liparulo V, Pica A, et al. Multidisciplinary approach to chest wall resection and reconstruction for chest wall tumors, a single center experience. J Thorac Dis 2017;9:5093-100. [Crossref] [PubMed]
- Dell'Amore A, Dolci G, Cassanelli N, et al. A massive post-sternotomy sternal defect treated by allograft sternal transplantation. J Card Surg 2012;27:557-9. [Crossref] [PubMed]
- Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J 2001;10:S96-101. [Crossref] [PubMed]
- LeGeros RZ. Properties of osteoconductive biomaterials: calcium phosphates. Clin Orthop 2002.81-98. [Crossref] [PubMed]
- Granero-Molto F, Weis JA, Longobardi L, et al. Role of mesenchymal stem cells in regenerative medicine: application to bone and cartilage repair. Expert Opin Biol Ther 2008;8:255-68. [Crossref] [PubMed]
- Wu Y, Chen N, Xu Z, et al. Application of 3D printing technology to thoracic wall tumor resection and thoracic wall reconstruction. J Thorac Dis 2018;10:6880-90. [Crossref] [PubMed]
- Tang H, Xu Z, Qin X, et al. Chest wall reconstruction in a canine model using polydioxanone mesh demineralizated bone matrix and bone marrow stromal cells. Biomaterials 2009;30:3224-33. [Crossref] [PubMed]
- Mattioli-Belmonte M, Montemurro F, Licini C, et al. Cell-Free Demineralized Bone Matrix for Mesenchymal Stem Cells Survival and Colonization. Materials (Basel) 2019;12:12. [Crossref] [PubMed]
(English Language Editor: John Ayric Gray, AME Publishing Company)
Cite this article as: Dell’Amore A, Ferrigno P, Pangoni A, Natale G, Faccioli E, Schiavon M, Mammana M, Comacchio GM, Rea F. The biological approach for sternal replacement: sternochondral allograft transplantation. Curr Chall Thorac Surg 2019;1:9.