Surgery for Pancoast tumors—the role of combined approaches
Introduction
Combined approaches in thoracic surgery are performed only in very selected patients (1,2). These rare technical options are not codified and executed only for difficult individual cases.
One of the possible indications for combined approaches is the surgical treatment of patients with Superior Sulcus tumors (Pancoast) infiltrating the anterior compartment of the thoracic inlet (3,4). Several anterior approaches have been developed to allow the complete resection of these tumors, mainly aimed at good exposure of the vascular structures of the thoracic inlet (5-10). Thanks to these techniques, tumors previously considered inoperable, can be resected with curative intent (11). The main drawbacks of the different anterior approaches are the damage to sternum, manubrium or clavicle and the poor access to lung hilum and/or spine; for the latter reason, a combined thoracotomy is frequently associated. In some selected patients with apical tumors infiltrating the anterior arch of first/second rib, without vascular involvement, an anterior approach has been proposed to expose the chest wall to be resected (12). In these rare situations we have developed an original technique (which we called “double step technique”) aimed at reducing the trauma and facilitating the surgical procedure (13), since all anterior approaches appear to be decidedly too traumatizing if the subclavian vessels are not completely exposed.
In the present study, we reviewed our experience in the treatment of patients with Pancoast tumors analyzing indications, techniques and oncological results and focusing on pros and cons of combined approaches. The secondary endpoint of the study was to explore the possible value of our “double step technique” to improve the surgical exposure in selected patients with large anterior Pancoast tumor.
Methods
A retrospective chart review was performed to identify patients submitted to surgery for infiltrating apical thoracic tumors at the Thoracic Surgery Units of the Perugia University (Santa Maria della Misericordia Hospital in Perugia and Santa Maria Hospital in Terni) over an eighteen-year period. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The present study obtained ethics approval from the institutional review board of Perugia University Hospital.All patients before surgery accepted the anonymous use of their clinical data for retrospective study. Notification regarding the accomplishment of the study was sent to the Local Ethics Committee (Comitato Etico Regionale dell’Umbria - Regione Umbria).
The patients were divided into groups in order to examine all the available clinical data including the surgical approaches carried out and the location of the tumors. The subgroup of combined approaches were further analyzed with the purpose of studying the surgical strategy adopted and the results of the “double steps” technique developed by the authors. Follow-up data were achieved by phone call or by cancer registry.
Statistical analysis
Descriptive statistics was performed using frequencies, percentages, frequency tables for categorical variables and mean ± standard deviation (SD) for quantitative variables.
Duration of overall survival (OS) was measured from the date of surgery until the date of death or the censor date of the last follow-up for vital.
Disease-free survival (DFS) was measured from the date of surgery until the date of recurrence or the censor date of the last follow-up for vital.
DFS and OS were calculated by the Kaplan-Meier method.
Statistical analysis were performed with STATA 15.2 (StataCorpLP, Collage Station TX, USA).
“Double step” technique
This technique, which has been described in detail elsewhere (13), can be synthesized in the following two steps:
- a short parasternal incision is used to cut the medial edge of the involved ribs at the required distance from to the sternum and to place multiple heavy stitches at the peristernal tissues. These stitches are temporarily left inside the chest and will be subsequently gathered for anchoring the chest wall prosthesis. The first step is concluded in about 30–40 minutes;
- a Shaw-Paulson thoracotomy is used to complete the en-bloc resection. The previously placed peristernal stitches, are collected and used for the medial fixation of a synthetic prosthesis (either mesh or soft patch) (Figure 1).
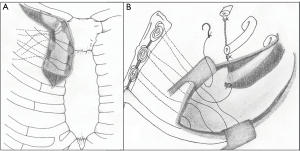 Figure 1 Schematic representation of the “double step technique”. (A) Through a limited parasternal incision, the medial edges of the first three ribs are interrupted. Some heavy non-absorbable stitches are placed at the peristernal level and temporarily left into the pleural cavity; (B) once completed the chest wall resection through the posterior approach, the previously placed peristernal stitches, are collected and used for the medial fixation of the prosthesis.
Figure 1 Schematic representation of the “double step technique”. (A) Through a limited parasternal incision, the medial edges of the first three ribs are interrupted. Some heavy non-absorbable stitches are placed at the peristernal level and temporarily left into the pleural cavity; (B) once completed the chest wall resection through the posterior approach, the previously placed peristernal stitches, are collected and used for the medial fixation of the prosthesis.
Patients
From July 2000 to July 2018, we identified 23 patients submitted to en bloc resection of the first ribs for Pancoast tumors, as defined by Kernstine et al. (apical lung tumors with evidence of involvement of I or II rib) (14). We observed 17 males and 6 females, with a mean age of 64.2 years (range, 45–75 years). All patients underwent disease staging with computed tomography (brain, chest and abdomen), bronchoscopy and tissue diagnosis via percutaneous sampling. 18FDG-Positron emission tomography was performed in all patients since 2005 (20 patients; 87.0%). Magnetic resonance imaging of the chest was performed in 10 patients (43.5%), mediastinoscopy in 2 (8.7%), EBUS-TBNA samples in 2 (8.7%) and bone scanning in 2 (8.7%). All patients complained of chest pain.
The preoperative diagnosis was non-small cell lung cancer in all patients. Clinical stage was cT3N0M0 in 17 patients, cT3N1M0 in 2 and cT4N0M0 in 4. No cN2 patients were considered for surgery. All but 5 patients (78.3%) underwent induction chemoradiotherapy with a median radiation dose of 45 Gy, or induction chemotherapy (2 patients; 8.7%). The decision not to perform any induction therapy was taken by the multidisciplinary team and was related to infection and cavitation of the tumor (4 cases; 17.4%) or to the patient’s refusal (1 patient; 4.3%).
Results
The extended posterior Shaw-Paulson approach (15) was performed in 14 patients (60.9%), the Grunenwald and Spaggiari transmanubrial approach (7) in 5 (21.7%) and the “double step” approach in 4 (17.4%).
A combined approach was performed in 9 (39.1%) patients: 5 in the group of transmanubrial incision and 4 in the “double step” group. The additional approach in the transmanubrial group was a thoracotomy (4 patients) and a three ports VATS lobectomy (one patient). En-bloc upper lobectomies were carried out in all patients but 2 (91.3%), who had a wedge resection through the posterior incision (8.7%). The wedge resections were performed because the tumor also infiltrated S6 or because the complete resection was questionable at the level of the spine and brachial plexus. The first three ribs were resected in 15 patients (65.2%), I–II rib in 4 (17.4%) and the first four ribs in 4 patients (17.4%). Disarticulation of the ribs from the transverse processes was performed in 6 patients (26.1%). The other anatomic structures involved in the en-bloc resection were: T1 nerve route (#2), T1-C8 nerve route (#1), sympathetic trunk (#2), subclavian vein (#1), subclavian artery (#1), thoracic duct (#1), phrenic nerve (#1), vertebral transverse processes (#2).
The prosthetic stabilization of the chest wall was performed in 5 (21.7%) patients (all patients in the “double step” group and 1 patient in the transmanubrial group). Heavy polypropylene meshes were used in 2 (8.7%) patients, polytetrafluoroethylene (PTFE) patch in 4 (17.4%). No patient submitted to chest wall stabilization experienced significant postoperative paradoxical breathing, despite the large thoracectomy they had undergone. Conversely, the paradoxical respiratory movements were important in 3 cases (13.0%) subjected to the transmanubrial approach (2 of these patients needed a formal tracheostomy to manage postoperative respiratory problems). Indeed, a not relevant post-operative paradoxical breathing was also observed in most patients of the posterior approach group. A mini-trach was placed in 2 (8.7%) patients (all of the posterior approach group) to treat mucous retention, whereas a formal tracheostomy was placed in other 2 patients (8.7%), both of the transmanubrial group.
No postoperative mortality was observed with a total complication rate of 48%, subdivided as follows: posterior approach 43%, anterior transmanubrial approach 40%, double step approach 50%.
Patient’s data and complications are reported in Table 1. N1 metastases were observed in 2 patients.
Table 1
| Operative variables | All patients | Surgical approach | ||
|---|---|---|---|---|
| Posterior access | Transmanubrial access + thoracotomy/VATS | Double-step | ||
| Total | 23 | 14 (60.9%) | 5 (21.7%) | 4 (17.4%) |
| Side | ||||
| Left | 15 (65.2%) | 9 (64.3%) | 5 (100%) | 1 (25%) |
| Right | 8 (34.8%) | 5 (35.7%) | – | 3 (75%) |
| Lung resection | ||||
| Lobectomy | 21 (91.3%) | 12 (85.7%) | 5 (100%) | 4 (100%) |
| Sublobar resection | 2 (8.7%) | 2 (14.3%) | – | – |
| Number of resected ribs | ||||
| I + II | 4 (17.4%) | 2 (14.3%) | 2 (40%) | – |
| I + II + III | 15 (65.2%) | 10 (71.4%) | 3 (60%) | 2 (50%) |
| I + II + III + IV | 4 (17.4%) | 2 (14.3%) | – | 2 (50%) |
| Procedure for vessel | ||||
| Subclavian vein resection | 1 (4.3%) | – | 1 (20%) | – |
| Subclavian artery reconstruction | 1 (4.3%) | – | 1 (20%) | – |
| Chest wall reconstruction | ||||
| Yes | 5 (21.7%) | – | 1 (20%) | 4 (100%) |
| No | 18 (78.3%) | 14 (100%) | 4 (80%) | – |
| Complications | ||||
| None | 11 (47.8%) | 8 (57.1%) | 1 (20%) | 2 (50%) |
| Hemothorax | 4 (17.4%) | 3 (21.4%) | 1 (20%) | – |
| Respiratory failure | 4 (17.4%) | 2 (14.3%) | 2 (40%) | – |
| Atrial fibrillation | 8 (34.8%) | 4 (28.6%) | 2 (40%) | 2 (50%) |
| Atelectasis | 5 (21.7%) | 3 (21.4%) | – | 2 (50%) |
| Minitrach/tracheostomy | 4 (17.4%) | 2 (14.3%) | 2 (40%) | – |
| Redo thoracotomy | 1 (4.3%) | 1 (7.1%) | – | – |
| Transient art edema | 1 (4.3%) | – | 1 (20%) | – |
| Hand paralysis | 1 (4.3%) | 1 (7.1%) | – | – |
| Chylothorax | 1 (4.3%) | – | 1 (20%) | – |
| Prolonged air leak | 1 (4.3%) | – | – | 1 (25%) |
VATS, video-assisted thoracoscopic technique.
The pathology report showed R1 resection in 6 patients (26.1%) and R0 resection in the remaining 17 patients (complete resection rate 74.0%). All patients in the double step group had a complete resection. Histology showed pulmonary adenocarcinoma (#13), large cell undifferentiated carcinoma (#4), squamous cell carcinoma (#4), sarcomatoid carcinoma (#1), high grade mucoepidermoid carcinoma (#1). One patient had a pathologic complete response. Disease and follow-up data of the 23 patients are summarized in Table 2.
Table 2
| Oncological variables | All patients | Surgical approach | ||
|---|---|---|---|---|
| Posterior access | Transmanubrial access + thoracotomy/VATS | Double-step | ||
| Total | 23 | 14 (60.8%) | 5 (21.7%) | 4 (17.4%) |
| Histology | ||||
| Adenocarcinoma | 13 (56.5%) | 9 (64.3%) | 2 (40%) | 2 (50%) |
| Squamous-cell carcinoma | 4 (17.4%) | 2 (14.3%) | 1 (20%) | 1 (25%) |
| Large-cell carcinoma | 4 (17.4%) | 3 (21.4%) | 1 (20%) | – |
| Sarcomatoid carcinoma | 1 (4.3%) | – | 1 (20%) | 1 (25%) |
| High-grade mucoepidermoid carcinoma | 1 (4.3%) | – | – | – |
| Residual tumor | ||||
| R0 | 17 (73.9%) | 10 (71.4%) | 3 (60%) | 4 (100%) |
| R1 | 6 (26.1%) | 4 (28.6%) | 2 (40%) | – |
| pTNM | ||||
| pT3N0M0 (IIB) | 17 (73.9%) | 12 (85.7%) | 3 (60%) | 2 (50%) |
| pT3N1M0 (IIIA) | 2 (8.7%) | – | 1 (20%) | 1 (25%) |
| pT4N0M0 (IIIA) | 4 (17.4%) | 2 (14.3%) | 1 (20%) | 1 (25%) |
| Neoadjuvant/adjuvant treatment | ||||
| None | 2 (8.7%) | 1 (7.1%) | – | 1 (25%) |
| Neoadjuvant | 7 (30.4%) | 4 (28.6%) | 2 (40%) | 1 (25%) |
| Adjuvant | 3 (13.0%) | 1 (7.1%) | 1 (20%) | 1 (25%) |
| Both | 11 (47.8%) | 8 (57.1%) | 2 (40%) | 1 (25%) |
| Pattern of recurrence | ||||
| None | 14 (60.9%) | 8 (57.1%) | 2 (40%) | 4 (100%) |
| Local | 6 (26.1%) | 5 (35.7%) | 1 (20%) | – |
| Distant | 3 (13.0%) | 1 (7.1%) | 2 (40%) | – |
| Survival | ||||
| Alive | 7 (30.4%) | 3 (21.4%) | 1 (20%) | 3 (75%) |
| Dead-cancer specific | 9 (39.1%) | 6 (42.9%) | 3 (20%) | – |
| Dead-other cause | 6 (26.1%) | 4 (28.6%) | 1 (20%) | 1 (25%) |
| Lost at FU | 1 (4.3%) | 1 (7.1%) | – | – |
FU, follow-up.
Median follow-up was 52.24 months with IQR 13.16–85.39 months (range, 1.48–164.7 months).
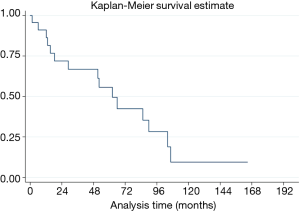
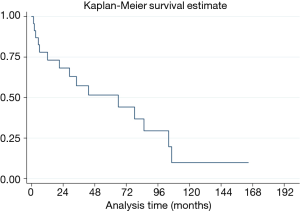
Median OS was 62.3 months (95% CI, 18.39–104.21 months). The 5-year OS was 55.6% (95% CI, 31.7–74.1%) (Figure 2).
The median overall disease-free survival was 65.9 months (95% CI, 12.1–104.2 months), whereas the 5-year DFS was 51.5% (95% CI, 28.2–70.7%) (Figure 3).
We then analyzed only the data of patients with R1 resection and we determined a median OS of 13.1 months (95% CI, 12.2–18.4 months) in this subset of patients.
The estimated proportion of deaths was 60.9% (95% CI, 39.1–79.0%) in the whole population of the study, whereas was 83.3% (95% CI, 23.0–98.8%) in the R1 group.
Discussion
The current potentially curative treatment of patients with Pancoast tumors is induction chemo-radiotherapy, followed by radical resection (16). Surgery plays a key role in the trimodality therapy with indications that must be discussed in a multidisciplinary context. Contraindications for surgery are well defined: distant metastasis, N2–N3 disease, involvement of the spinal canal, involvement of more than 50% of the vertebral bodies, esophageal or tracheal invasion and infiltration of the brachial plexus above T1 (17). Involvement of C8 is a negative prognostic factor (18) and extending resection above T1 level entails severe functional problems to the hand, as we observed in one patient. In the event of massive involvement of the subclavian vessels, of the brachial plexus and of the spine, surgery can be attempted in high volume centers but R0 resection is frequently questionable (19).
Surgical techniques for Pancoast tumors cannot be standardized, considering the wide variability of clinical situations. For the purpose of the surgical approach, it is necessary to weigh different parameters, related to the site and the size of the tumor, to the structures potentially involved in resection and to the experience of the surgeon. The thoracic inlet has been divided into three compartments: anterior (between sternum and anterior scalene muscle), middle (between anterior and middle scalene muscles), posterior (behind the middle scalene muscle) (20). In 2012 De Perrot and Rampersaud (21) divided the thoracic inlet into five anatomical zones, each of which requires a specific consideration for the surgical approaches. In our opinion, this study represents a significant contribution for the comprehension of the complicated surgical anatomy of this area. However, Pancoast tumors are so uncommon that it is impossible for most thoracic surgeon to gather meaningful experience with all the various approaches described in the literature. Conversely, it is essential to be familiar with the basic approaches, as well as the pros and cons of the possible combined incisions (18).
The posterior approach is often performed for the treatment of patients with Pancoast tumors (3,18,22), even if it is not suitable in all situations. Such incision allows good exposure of the posterior chest wall, including vertebrae and brachial plexus (22). Also the unforeseen infiltration of the subclavian artery could be treated from below, but the vascular exposure is not ideal (18). Many Superior Sulcus tumors not requiring the surgical exposure of the subclavian vessels may be managed by this approach, even in the case of anterior infiltration of the chest wall (18). Technical problems can occur in the management of very bulky tumors infiltrating the first ribs anteriorly, as the large mass can hide the anterior limits of parietal infiltration, if approached from the back. Tatsumura proposed extending the posterior incision anteriorly and upwards to the nipple, in order to expose the sternoclavicular joints (23). This approach is rarely chosen because of its invasiveness. The hemiclamshell approach has been also proposed (24) but it is rarely performed and is equally traumatizing. On the other hand, an anterior approach seems to be the most reasonable choice for this group of patients.
The various anterior clavicular-manubrial-sternal approaches allow good exposure of the vascular structures of the thoracic inlet and are indicated in case of subclavian vessels involvement. One of the most used anterior access in clinical practice is the transmanubrial approach, proposed by Grunenwald and Spaggiari in 1997, which has the great advantage of avoiding clavicle resection, reducing this way functional and cosmetic problems (7); for this reason it is our first choice for tumors infiltrating the subclavian vessels, although our experience is limited. Regardless of the damage to sternum or clavicle, most anterior accesses provide poor exposure to lung hilum and/or spine. Actually, they entail that lobectomy and mediastinal dissection must be performed through the hole resulting from the chest wall resection, through further enlargement of the incision or through an additional approach (3,5,8). As correctly stated by Parissis et al. (25) the need to perform an additional posterolateral thoracotomy eliminates the advantage of the routine use of the anterior-manubrial sternal approach. Any anterior access, damaging the sternum or clavicle, is futile if the complete exposure of subclavian vessels is not required. Furthermore, no anterior incision allows for the correct handling of the mass, especially when a large tumor attached to the resected ribs is released within the chest. These reasons led us to develop the “double step” technique, which we believe can be useful in selected cases.
Combined approaches can be required for adequate surgical exposure in patients with tumors involving the anterior compartment of the thoracic inlet. Some authors deem such surgical choices to be not ideal because they increase the surgical trauma and may cause respiratory complications and wound healing problems (26,27). Combined approaches were performed in 39.1% of patients in our series, mostly as a deliberate choice. Morbidity rate was high (even though most were minor complications) and did not differ between patients with single and combined access. In our opinion in many patients with “anterior” Pancoast tumors the advantages of an additional incision overcome the disadvantages.
The recent introduction of VATS has renewed interest in combined accesses for Pancoast tumors and has provided new technical solutions: indeed the VATS approach can be used to carry out the pulmonary lobectomy, after the anterior dissection has been completed (28-30). In our experience such technique has only been possible in one patient with a tumor infiltrating the subclavian vein and the first intercostal space. On the other hand, this data must be analyzed in light of the fact that, in our department, VATS lobectomies entered routine activity only since 2014. We believe that, when feasible, VATS allows a significant reduction in surgical trauma, although a combined VATS approach only makes sense if the surgical resection specimen is not so bulky that it cannot be easily moved inside the chest.
As a general rule, we preferred the extended posterior approach in all patients but those with tumor infiltrating anteriorly the chest wall. The transmanubrial approach was chosen in case of subclavian vessels involvement, while the “double step” technique was preferred if the anterior infiltration was only limited to the chest wall.
We adopted the “double step” technique in Pancoast tumor patients with a large anterior chest wall infiltration, provided that the subclavian vessels were not involved. In this subgroup of patients this technique proved to be rapid and simple and allowed clear margins of resection to be obtained with reduced trauma (the added anterior incision is comparable to that of an anterior mediastinotomy). Furthermore, the positioning of the prosthesis, recommended for the large anterior thoracectomy, has been greatly facilitated.
The prosthetic reconstruction is not carried out after most Pancoast tumor resections. However, we want to underline the advantages of the prosthetic reconstruction in patients submitted to a large anterior thoracectomy. Postoperative paradoxical breathing is negligible when the scapula cover the residual defect (31); conversely, in case of large anterior chest wall defect, the prosthetic reconstruction avoid important respiratory movements of the chest wall, so reducing morbidity and improving functional results.
Regarding the oncological perspective, our results are similar to those observed in the recent Literature. In historical reports, when bimodality therapy (radiotherapy plus surgery) was the standard of care, 5-year OS ranged between 25–35% (6,32,33). In the past two decades, with the introduction of trimodality therapy and the refinement of surgical techniques, an improvement of survival rates was highlighted, with a 5-year OS of 36–56% in more recent studies (34-37).
As a complete resection of the neoplasm represents one of the strongest prognostic factors of OS and DFS, along with T status and complete pathological response to therapy, every effort should be made to obtain pathologic negative margin (34,35,38). This may represent a challenging target, considering that even in high-volume centers, only few patients are submitted to surgical treatment of Pancoast tumor. Indeed our “double-step” technique, as described above, facilitates the surgical procedure and aids the surgeon to obtain a complete resection.
Conclusions
The surgical approach for patients with Pancoast tumors is widely variable and depends on the extent of local infiltration, tumor size, technical choice and experience of the surgeon.
The basic approach is the posterior extended incision, which is ideal for posterior and most posteromedial tumors.
Anterior tumors infiltrating the subclavian vessels can be treated through a number of incisions: the transmanubrial approach entails the advantage of clavicular sparing and offers excellent exposure of subclavian vessels/brachial plexus.
In anteriorly located tumors without vascular involvement sternal or clavicular damage should be avoided.
An additional approach is often indicated for patients with “anterior Pancoast tumors” and this technical choice may become decisive to achieve safe and complete resection in selected patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Francesco Puma and Hon Chi Suen) for the series “Surgical Management of Chest Wall Tumors” published in Current Challenges in Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts.2019.12.04/coif). The series “Surgical Management of Chest Wall Tumors” was commissioned by the editorial office without any funding or sponsorship. FP served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Current Challenges in Thoracic Surgery from March 2019 to March 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that question related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The present study obtained ethics approval from the institutional review board of Perugia University Hospital. All patients before surgery accepted the anonymous use of their clinical data for retrospective study. Notification regarding the accomplishment of the study was sent to the Local Ethics Committee (Comitato Etico Regionale dell’Umbria - Regione Umbria).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Scarnecchia E, Liparulo V, Capozzi R, et al. Multidisciplinary approach to chest wall resection and reconstruction for chest wall tumors, a single center experience. J Thorac Dis 2017;9:5093-100. [Crossref] [PubMed]
- Puma F, Cardini CL, Passalacqua G, et al. Preoperative embolization in surgical management of giant thoracic sarcomas. Eur J Cardiothorac Surg 2008;33:127-9. [Crossref] [PubMed]
- Alifano M, D'Aiuto M, Magdeleinat P, et al. Surgical treatment of superior sulcus tumors: results and prognostic factors. Chest 2003;124:996-1003. [Crossref] [PubMed]
- Foroulis CN, Zarogoulidis P, Darwiche K, et al. Superior sulcus (Pancoast) tumors: current evidence on diagnosis and radical treatment. J Thorac Dis 2013;5:S342-58. [PubMed]
- Dartevelle P, Levasseur P, Rojas-Miranda A, et al. Combined cervical and thoracic approach to the removal of tumours responsible for the Pancoast and Tobias syndrome (author’s transl). Nouv Presse Med 1981;10:1051-4. [PubMed]
- Dartevelle PG, Chapelier AR, Macchiarini P, et al. Anterior transcervical-thoracic approach for radical resection of lung tumors invading the thoracic inlet. J Thorac Cardiovasc Surg 1993;105:1025-34. [Crossref] [PubMed]
- Grunenwald D, Spaggiari L. Transmanubrial osteomuscular sparing approach for apical chest tumors. Ann Thorac Surg 1997;63:563-6. [Crossref] [PubMed]
- Masaoka A, Ito Y, Yasumitsu T. Anterior approach for tumor of the superior sulcus. J Thorac Cardiovasc Surg 1979;78:413-5. [Crossref] [PubMed]
- Nazari S. Transcervival approach (Dartevelle technique) for resection of lung tumors invading the thoracic inlet, sparing the clavicle. J Thorac Cardiovasc Surg 1996;112:558-60. [Crossref] [PubMed]
- Nomori H, Nara S, Horio H. Modified trap-door thoracotomy for malignancies invading the subclavian and innominate vessels. Thorac Cardiovasc Surg 1995;43:204-7. [Crossref] [PubMed]
- Spaggiari L, D'Aiuto M, Veronesi G et al. Anterior approach for Pancoast tumor resection. Multimed Man Cardiothorac Surg 2007;2007:mmcts.2005.001776.
- Bobbio A, Strano S, Alifano M. Resection of superior sulcus cancers (anterior approach). Shanghai Chest 2017;1:12. [Crossref]
- Puma F, Vannucci J, Scarnecchia E, et al. Original “double-step” technique for large superior sulcus tumors invading the anterior chest wall without subclavian vessels involvement. J Thorac Dis 2018;10:S1850-4. [Crossref] [PubMed]
- Kernstine KH, Moon J, Kraut MJ, et al. Trimodality therapy for superior sulcus non-small cell lung cancer: Southwest Oncology Group-Intergroup Trial S0220. Ann Thorac Surg 2014;98:402-10. [Crossref] [PubMed]
- Shaw RR, Paulson DL, Kee JL. Treatment of Superior Sulcus Tumor by Irradiation Followed by Resection. Ann Surg 1961;154:29-40. [Crossref] [PubMed]
- Ettinger DS, Aisner DL, Wood DE, et al. National Comprehensive Cancer Network. NCCN Guidelines Insights: Non-small Cell Lung Cancer, Version 5.2018. J Natl Compr Canc Netw 2018;16:807-21. [Crossref] [PubMed]
- Detterbeck FC. Changes in the treatment of Pancoast tumors. Ann Thorac Surg 2003;75:1990-7. [Crossref] [PubMed]
- Nicastri DG, Swanson SJ. Pros and Cons of Anterior and Posterior Approaches to Pancoast Tumors: Posterolateral Superior Sulcus Tumor Resections. Oper Tech Thorac Cardiovasc Surg 2006;11:141-53. [Crossref]
- Tamura M, Hoda MA, Klepetko W. Current treatment paradigms of superior sulcus tumours. Eur J Cardiothorac Surg 2009;36:747-53. [Crossref] [PubMed]
- Rusch VW. Management of Pancoast tumors. Lancet Oncol 2006;7:997-1005. [Crossref] [PubMed]
- de Perrot M, Rampersaud R. Surgical approaches to apical thoracic malignancies. J Thorac Cardiovasc Surg 2012;144:72-80. [Crossref] [PubMed]
- Kent MS, Bilsky MH, Rusch VW. Resection of superior sulcus tumors (posterior approach) Thorac Surg Clin 2004;14:217-28. [Crossref] [PubMed]
- Tatsumura T, Sato H, Mori A, et al. A new surgical approach to apical segment lung disease, including carcinomas and inflammatory diseases. J Thorac Cardiovasc Surg 1994;107:32-6. [Crossref] [PubMed]
- Korst RJ, Burt ME. Cervicothoracic tumors: results of resection by the ‘hemiclamshell’ approach. J Thorac Cardiovasc Surg 1998;115:286-94. [Crossref] [PubMed]
- Parissis H, Young V. Treatment of Pancoast tumors from the surgeons prospective: re- appraisal of the anterior-manubrial sternal approach. J Cardiothorac Surg 2010;5:102. [Crossref] [PubMed]
- Zairi F, Sunna T, Liberman M, et al. Single Posterior Approach for En-Bloc Resection and Stabilization for Locally Advanced Pancoast Tumors Involving the Spine: Single Centre Experience. Asian Spine J 2016;10:1047-57. [Crossref] [PubMed]
- Marulli G, Battistella L, Mammana M, et al. Superior sulcus tumors (Pancoast tumors). Ann Transl Med 2016;4:239. [Crossref] [PubMed]
- Truin W, Siebenga J, Belgers E, et al. The role of video-assisted thoracic surgery in the surgical treatment of superior sulcus tumors. Interact Cardiovasc Thorac Surg 2010;11:512-4. [Crossref] [PubMed]
- Linden PA. Video-assisted anterior approach to Pancoast tumors. J Thorac Cardiovasc Surg 2010;140:e38-9. [Crossref] [PubMed]
- Nakajima T, Watanabe A, Nakazawa J, et al. Transmanubrial approach with video-assisted thoracoscopic surgery for left superior sulcus tumour with dense adhesion after replacement of descending thoracic aorta. Interact Cardiovasc Thorac Surg 2012;14:906-8. [Crossref] [PubMed]
- Puma F, Vannucci J. Chest wall resection/reconstruction for tumors. In: Mathisen DJ, Morse CR, Fischer JE, editors. Master Techniques in Surgery. Thoracic Surgery: Transplantation, Tracheal Resections, Mediastinal tumors, Extended Thoracic Resections. Wolters Kluwer, Philadelphia, 2015;149-72.
- Ginsberg RJ, Martini N, Zaman M, et al. Influence of surgical resection and brachytherapy in the management of superior sulcus tumor. Ann Thorac Surg 1994;57:1440-5. [Crossref] [PubMed]
- Shahian DM, Neptune WB, Ellis FH Jr. Pancoast tumors: improved survival with preoperative and postoperative radiotherapy. Ann Thorac Surg 1987;43:32-8. [Crossref] [PubMed]
- Rusch VW, Giroux DJ, Kraut MJ, et al. Induction chemoradiation and surgical resection for superior sulcus non-small-cell lung carcinomas: long-term results of Southwest Oncology Group Trial 9416 (Intergroup Trial 0160). J Clin Oncol 2007;25:313-8. [Crossref] [PubMed]
- Kunitoh H, Kato H, Tsuboi M, et al. Phase II trial of preoperative chemoradiotherapy followed by surgical resection in patients with superior sulcus non-small-cell lung cancers: report of Japan Clinical Oncology Group trial 9806. J Clin Oncol 2008;26:644-9. [Crossref] [PubMed]
- Fischer S, Darling G, Pierre AF, et al. Induction chemoradiation therapy followed by surgical resection for non-small cell lung cancer (NSCLC) invading the thoracic inlet. Eur J Cardiothorac Surg 2008;33:1129-34. [Crossref] [PubMed]
- Marulli G, Battistella L, Perrissinotto E, et al. Results of surgical resection after induction chemoradiation for Pancoast tumours. Interact Cardiovasc Thorac Surg 2015;20:805-11. [Crossref] [PubMed]
- Goldberg M, Gupta D, Sasson AR, et al. The surgical management of superior sulcus tumors: a retrospective review with long-term follow-up. Ann Thorac Surg 2005;79:1174-9. [Crossref] [PubMed]
Cite this article as: Puma F, Gili A, Cagini L, Vinci D, Matricardi A, Berti V, Italiani A, Potenza R, Ceccarelli S. Surgery for Pancoast tumors—the role of combined approaches. Curr Chall Thorac Surg 2020;2:4.