
When magnetic resonance imaging is needed: malignant pleural mesothelioma with transdiaphragmatic infiltration and localized appearance—a case report
Introduction
Malignant pleural mesothelioma (MPM) is an aggressive tumor of the mesothelial lining of the chest and is most commonly associated with an occupational exposure to asbestos (1). In most cases, MPM shows a diffuse growth pattern and spreads widely across the pleural surface (2). However, sporadic cases of localized MPM with a bulky, circumscribed appearance have been initially described over 40 years ago (3). While both, localized and diffuse MPM, display identical histological, immunohistochemical and molecular characteristics, localized MPM mostly show a less aggressive clinical course and the role of chemotherapy after localized surgical resection remains controversial (4-7). In order to differentiate between a localized MPM and the rare variant of a diffuse MPM with a dominant, circumscribed mass, a complete examination of the pleural cavity is required (5).
Computed tomography (CT) in general is the standard of reference for primary assessment of tumor extend. For tumor staging 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography-computed tomography (PET-CT) is used. However, there are different studies showing that magnetic resonance tomography (MRI) goes beyond CT concerning the depiction of diaphragmatic and chest wall infiltration.
Here, we report the rare case of a diffuse MPM with radiologically localized appearance and transdiaphragmatic infiltration. The atypical presentation featuring a solitary tumor bulk and the subsequent histological confirmation of a disseminated disease add to the educational value of this case. In addition, the surgical planning was primarily performed according to the findings of the preoperative MRI. The role of MRI for preoperative planning has not been previously described and clinical evidence is therefore poor. A timeline of each diagnostic or therapeutic intervention is depicted in Figure 1. We present the following case in accordance with the CARE reporting checklist (available at https://ccts.amegroups.com/article/view/10.21037/ccts-20-41/rc).
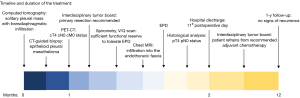
Case presentation
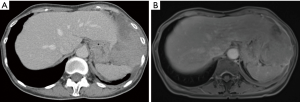
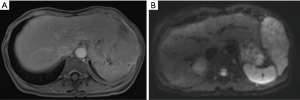
A 58-year-old female patient from east Asia presented with a recent onset of postprandial gastric discomfort, night sweats and weight loss. While both gastroscopy and colonoscopy showed not pathological findings, a CT revealed a solitary, partly solid and partly cystic pleural mass, measuring 9.4 cm × 5.8 cm. The tumor was located on the left diaphragm with transdiaphragmal infiltration and slight displacement of the stomach, the spleen and the liver (Figure 2). No pleural or peritoneal effusion was present. Upon CT-guided transthoracic biopsy, a MPM with epithelioid differentiation was diagnosed. Immunohistochemistry showed a positivity for CK5/6, CK7 and WT1. In the immunostaining for BAP-1 no retained expression was identified. The patient had no suggestive occupational history of exposure to asbestos. Following further staging by PET-CT, no other pleural or abdominal lesions and no mediastinal lymphadenopathy were identified and a clinical T4 N0 M0 status was defined. The mesothelioma showed a 18F-FDG accumulation with maximum standardized uptake value (SUVmax) of 15.2. The calculated total tumor volume was estimated at 250 mL. Due to the localized finding without signs of diffuse pleural dissemination or other distant manifestations, a direct resection was recommended in our interdisciplinary tumor board. Pre-operative spirometry showed sufficient functional reserve [FEV1: 1.86 L (84% of set)] and the perfusion of the diseased left lung (V/Q-scan) was only moderately reduced at 37%. For preoperative planning a MRI of the chest was performed, showing the transdiaphragmatic growth and infiltration into chest wall (Figures 2-4). The tumor showed a moderate contrast-uptake but a high signal on diffusion-weighted image, which is typical for epitheleoid mesothelioma. Furthermore, the MR showed an infiltration into the fascia endothoracica of the chest wall (Figure 4).

The patient underwent an extended pleurectomy and decortication (EPD) with resection of pericardium and diaphragm, followed by a systematic lymphadenectomy of the mediastinal lymph nodes (Appendix 1). The pericardium was reconstructed with an acellular biological patch. After resection of the diaphragm encompassing the bulky tumor, the peritoneal cavity was opened and inspected. Apart from minor adhesions of the tumor bulk to the greater omentum, no infiltration into the visceral organs was seen macroscopically. The adhesive region of the omentum was resected and the diaphragm was reconstructed with a Gore-Tex® Dualmesh® biomaterial patch (W.L. Gore & Associates Inc., Flagstaff, AZ, USA). Single wedge-resections were necessary in two sites where an infiltration into the lung parenchyma was suspected. While the histological analysis of the partially resected greater omentum confirmed no malignant process, infiltrations of the epithelioid pleural mesothelioma were verified in the specimens of both visceral- and parietal pleura, pericardium and lung parenchyma, corresponding to a pathological T4 N0 M0 status.
The patient was discharged after an uneventful recovery on the 11th postoperative day. Within the intended multimodality treatment approach, an adjuvant chemotherapy with platinum and pemetrexed was planned. However, the patient wished to refrain from chemotherapy. To date, regular clinical and radiological follow-up 1 year after surgery shows no signs of relapse.
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Comments
Our case demonstrates a diffuse MPM with a dominant mass on the left hemidiaphragm and transdiaphragmatic infiltration. The radiological findings were at first not suggestive for an MPM and differential diagnoses included lymphoma, gastrointestinal stromal tumor or mesenchymal tumors. After histological confirmation of an epithelioid pleural malignancy, a localized MPM was suspected and the patient was therefore referred directly for operative resection, without neoadjuvant chemotherapy, our usual concept of MPM treatment. The intraoperative examination of the complete pleural cavity unfortunately revealed additional nodular lesions on the pericardium, the parietal and the visceral pleura, corresponding to a disseminated disease. Consequently, an adjuvant chemotherapy with platinum and pemetrexed was recommended by our interdisciplinary tumor board.
Localized MPM are assumed to account for approximately 0.5% of all MPM cases (5). Histologically and immunohistochemically, localized MPM are indistinguishable from diffuse MPM (4,5). In a recent study by Hung et al., localized MPM was furthermore found to harbor similar genomic alterations such as BAP1 mutations, deletions in CDKN2A and NF2, TRAF7-mutations and genomic near-haploidization (5). Contrary to diffuse MPM, the association with asbestos exposure and male sex is not as strong and the clinical course is often less aggressive (4). In the largest case series to date by Allen et al., 48% of all patients with localized MPM undergoing isolated surgical resection were free from recurrence after a mean follow-up of 4.8 years (4). Hence, an aggressive treatment and surgical excision is recommended. However, the role of chemotherapy has yet to be assessed in these patients (5-7). In contrast, international guidelines are established for the initial treatment of diffuse MPM and a multimodality concept including (neo)- or adjuvant chemotherapy with platinum and anti-folate doublet, followed by macroscopic complete resection has been shown to extend the overall- and disease-free survival (8-13).
Regarding imaging assessment, chest X-ray is usually the first-line diagnostic approach of MPM showing suspicious unilateral pleural effusion, unilateral pleural thickening with or without thickening pleural fissures, multiple masses with peripheral distribution or loss of volume in the involved hemithorax (14). Contrast enhanced CT of the chest represents the gold standard to evaluate MPM associated features like circumferential pleural thickening (pleural rind), thickened mediastinal pleura, nodular or irregular pleural thickening, infiltration of chest wall, diaphragm, mediastinum or pericardium and lymph nodes in extra pleural fat tissue (15). Although MRI is not routinely performed in patients with MPM, it has excellent soft tissue contrast and is therefore superior to CT for assessing chest wall and diaphragm invasion and revealing endothoracic fascia involvement (Figure 3) (16). Furthermore, recent studies have shown promising benefits of diffusion-weighted MR imaging (DWI) to differentiate malignant pleural disease from benign pleural alterations (Figure 4) (17) and delayed phase enhancement MRI and early contrast-enhancement MRI are on the verge of significantly improve the accuracy of early diagnosis as well as staging and therapy response assessment of MPM (18,19). Meanwhile, 18F-FDG-PET/CT surpasses CT and MRI in N and M staging of MPM (but not for T-staging) and is beneficial for evaluating treatment response and detection of recurrent disease (20).
While diffuse MPM most commonly presents as a uniform thickening of the pleura, rare cases featuring a circumscribed, bulky lesion may mimic a localized MPM (4,5). Our case report displays that their distinction may be difficult based on the preoperative radiological or bioptic findings. The case therefore highlights the importance of a meticulous intraoperative examination of the pleural cavity in patients where a localized MPM is suspected. The direct comparability between radiological findings from PET-CT and MRI with intraoperative, macroscopic findings and histological, microscopic results substantiate this conclusion in our case. The circumstance that localized pleural mesothelioma forms a rare disease makes it difficult to confirm our findings with other cases. The limitation of this case report are therefore its limited external validity and generalizability.
Conclusions
This case of a localized variant of epithelioid mesothelioma with transdiaphragmatic growth highlights the importance of MRI for a thorough preoperative assessment and of a careful intraoperative examination of the pleural cavity in these patients.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://ccts.amegroups.com/article/view/10.21037/ccts-20-41/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts-20-41/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Delgermaa V, Takahashi K, Park EK, et al. Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull World Health Organ 2011;89:716-24, 724A-724C.
10.2471/BLT.11.086678 10.2471/BLT.11.086678 - Cardinale L, Ardissone F, Gned D, et al. Diagnostic Imaging and workup of Malignant Pleural Mesothelioma. Acta Biomed 2017;88:134-42. [PubMed]
- Okike N, Bernatz PE, Woolner LB. Localized mesothelioma of the pleura: benign and malignant variants. J Thorac Cardiovasc Surg 1978;75:363-72. [Crossref] [PubMed]
- Allen TC, Cagle PT, Churg AM, et al. Localized malignant mesothelioma. Am J Surg Pathol 2005;29:866-73. [Crossref] [PubMed]
- Hung YP, Dong F, Dubuc AM, et al. Molecular characterization of localized pleural mesothelioma. Mod Pathol 2020;33:271-80. [Crossref] [PubMed]
- Nakano T, Hamanaka R, Oiwa K, et al. Localized malignant pleural mesothelioma. Gen Thorac Cardiovasc Surg 2012;60:468-74. [Crossref] [PubMed]
- Tanzi S, Tiseo M, Internullo E, et al. Localized malignant pleural mesothelioma: report of two cases. J Thorac Oncol 2009;4:1038-40. [Crossref] [PubMed]
- Cao C, Tian D, Manganas C, et al. Systematic review of trimodality therapy for patients with malignant pleural mesothelioma. Ann Cardiothorac Surg 2012;1:428-37. [PubMed]
- Cao C, Tian D, Park J, et al. A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer 2014;83:240-5. [Crossref] [PubMed]
- Kucukoner M, Ali Kaplan M, Inal A, et al. Clinical characteristics, treatment and survival outcomes in malignant pleural mesothelioma: an institutional experience in Turkey. J BUON 2014;19:164-70. [PubMed]
- Rusch V, Baldini EH, Bueno R, et al. The role of surgical cytoreduction in the treatment of malignant pleural mesothelioma: meeting summary of the International Mesothelioma Interest Group Congress, September 11-14, 2012, Boston, Mass. J Thorac Cardiovasc Surg 2013;145:909-10.
- Baas P, Fennell D, Kerr KM, et al. Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v31-9. [Crossref] [PubMed]
- Opitz I, Weder W. Pleural mesothelioma: is the surgeon still there? Ann Oncol 2018;29:1710-7. [Crossref] [PubMed]
- Wechsler RJ, Rao VM, Steiner RM. The radiology of thoracic malignant mesothelioma. Crit Rev Diagn Imaging 1984;20:283-310. [PubMed]
- Metintas M, Ucgun I, Elbek O, et al. Computed tomography features in malignant pleural mesothelioma and other commonly seen pleural diseases. Eur J Radiol 2002;41:1-9. [Crossref] [PubMed]
- Heelan RT, Rusch VW, Begg CB, et al. Staging of malignant pleural mesothelioma: comparison of CT and MR imaging. AJR Am J Roentgenol 1999;172:1039-47. [Crossref] [PubMed]
- Coolen J, De Keyzer F, Nafteux P, et al. Malignant pleural mesothelioma: visual assessment by using pleural pointillism at diffusion-weighted MR imaging. Radiology 2015;274:576-84. [Crossref] [PubMed]
- Patel AM, Berger I, Wileyto EP, et al. The value of delayed phase enhanced imaging in malignant pleural mesothelioma. J Thorac Dis 2017;9:2344-9. [Crossref] [PubMed]
- Tsim S, Humphreys CA, Cowell GW, et al. Early Contrast Enhancement: A novel magnetic resonance imaging biomarker of pleural malignancy. Lung Cancer 2018;118:48-56. [Crossref] [PubMed]
- Cardinale L, Ardissone F, Gned D, et al. Diagnostic Imaging and workup of Malignant Pleural Mesothelioma. Acta Biomed 2017;88:134-42. [PubMed]
Cite this article as: Werner RS, Fischer G, Inci I, Opitz I, Frauenfelder T. When magnetic resonance imaging is needed: malignant pleural mesothelioma with transdiaphragmatic infiltration and localized appearance—a case report. Curr Chall Thorac Surg 2021;3:30.


