
恶性胸膜间皮瘤的治疗及进展
引言
尽管 90% 的病例是恶性胸膜间皮瘤 (malignant pleural mesothelioma, MPM) ,恶性间皮瘤还可发生于腹膜、心包或鞘膜等处[1]。发生MPM 的风险与石棉暴露呈剂量依赖性,85%~90%的男性患者有职业性石棉暴露,而大多数女性患者存在间接性职业性石棉暴露[1];其他病因包括放疗、慢性胸膜炎、化学致癌物,以及1963 年以前可能被猿猴病毒污染的脊髓灰质炎疫苗[2],以及可能的遗传因素 [3,4]。 MPM 的典型临床表现为50岁至70岁的男性,出现继发于胸腔积液的呼吸困难或因肿瘤局部侵犯引起的非胸膜炎性胸壁疼痛 [3],80%~ 95% 的患者起病隐匿,胸部X线片有胸腔积液表现 [2]。英国的MPM患病率最高,由于潜伏期长,预计高收入国家的MPM发病率将在石棉法规实施后40年左右达到峰值 [2];而低、中收入国家的MPM发病率已被低估,且将继续上升[1]。据信,美国约有40%的劳动力在1940年至1979年间受到石棉暴露 [3]。尽管如此,MPM 仍然是一种罕见且致命的疾病,只有2%~10%的暴露人员会最终发生MPM,如果不进行治疗,预期生存时间仅6个月 [3]。然而,由于包括新一代测序在内的新诊断技术的应用,新的潜在治疗靶点不断发现,MPM 的治疗前景正在迅速变化[5]。因此,本文旨在全面综述恶性胸膜间皮瘤目前的治疗方案及新兴的治疗方法。我们根据Narrative Review Checklist提供本文链接(地址:http://dx.doi.org/10.21037/ccts-20-112)。
研究方法
通过PubMed 进行检索,关键词为malignant pleural mesothelioma 和 mesothelioma;时间限定为 2000 年至 2020 年 4 月;语言限定为英语和法语;文献类型包括高质量随机对照试验、队列和横断面研究以及系统评价和叙述性文献综述。北美和欧洲的协会指南也被纳入并参考。排除主题不是关于胸膜恶性间皮瘤的文献以及病例报告。
目前治疗方法
外科手术
早在1922年,在MPM被发现与肺癌不同之前,Eiselsberg 就提倡对弥漫性 MPM 进行根治性手术,他主张进行胸膜切除术,根治性胸膜全肺切除术或胸膜外全肺切除术 (extrapleural pneumonectomy, EPP,切除范围包括胸膜、肺、淋巴结、同侧心包及膈肌)成为20世纪50 年代可选择的治疗方法之一 [6,7]。然而,鉴于其糟糕的治疗效果,很少有人提倡这种过于激进的手术干预,姑息治疗仍然是标准疗法。直到Butchart等人证实29例患者中有2例长期治愈,尽管住院死亡率达31%,而并发症发生率达44.8% [6]。
随着时间的推移,通过对患者进行筛选以及围手术期管理水平的提高,手术效果也有所改善(目前围手术期死亡率为8%,而20世纪70年代的围手术期死亡率为33%)[8],尽管生存率还是以月而不是年来衡量,而且在过去40余年里MPM生存期没有任何显着改善 [9,10]。与其他肿瘤切除术目标相反,鉴于达到R0切除技术难度以及术后并发症的高发生率,MPM 手术的目标是进行最大程度的细胞减灭术并获得肉眼可见肿瘤的彻底切除(R1切除),再辅以其他局部和全身治疗 [7,9,11-14]。然而,手术切除在 MPM 治疗中的作用一直存在争议,许多协会建议在临床试验之外不要对MPM进行手术干预[15,16],而其他协会则支持对早期MPM进行手术切除[11,17-20]。即使在胸外科医生中,对手术治疗MPM的实践和理念也普遍存在差异 [21]。各种研究结果相互矛盾、疾病和治疗的异质性,以及缺乏高质量的随机对照试验 (randomized control trial, RCT)可以解释不同建议和指南间相互矛盾的观点 [22]。对来自SEER (Surveillance, Epidemiology, and End Results) 数据库的数据分析表明,细胞减灭术与生存期延长相关,因此作者建议以手术为中心的综合治疗应作为治疗基石 [10]。事实上,手术干预可能带来 9 个月的生存获益,但其相关的并发症发生率和死亡率很高 [23]。即便是老年患者,如果选择得当,手术也可能带来生存益处,尽管很少有人真正适合手术 [24]。
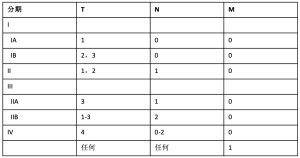
在支持手术治疗MPM的学者中,对于最佳的手术方案仍缺乏共识 [25],而且没有任何协会在这方面持明确立场 [9,26,27]。坚定支持EPP的学者认为其可以达到肿瘤最大限度的切除,尤其是对于早期患者(了解目前分期信息请参阅表1, 表2) [7,13,29]。另一个支持 EPP 的论点是全肺切除后允许高剂量的辅助放疗 [14]。回顾性数据显示,EPP可以使特定患者获得一定的生存获益,尤其是在新辅助或辅助放化疗的患者中 [30],无进展生存期 (progression-free survival, PFS) 可能会延长 [31]。然而,EPP 与高并发症发生率 (25%) 和高死亡率 (4%~15%)相关[15]。事实上,2011 年MARS (Mesothelioma and Radical Surgery) 试验比较了 EPP 与非EPP,因EPP术后并发症和30天死亡率高而不被推荐,尽管该试验是一个可行性研究,效力不足。考虑到这一点,其他专家主张病变范围切除较少的胸膜切除剥脱术 (pleurectomy and decortication,PD)(也称为扩大型 PD),术中切除脏层和壁层胸膜,偶尔切除心包和膈肌,但是不切除肺 [7,13]。一些研究发现,这种手术的术后并发症和死亡率显著降低,而生存结局相当 [8,31,33,34]。此外,一些系统评价发现 PD 更有生存获益,这可能是由围术期死亡率降低造成的[35,36],而且 PD 术后生活质量更高 [34,37],导致如今许多学者支持 PD治疗MPM。然而,其他的回顾性研究、系统评价和倾向性匹配比较表明,这两种手术在并发症发生率和死亡率方面没有差异 [38-40]。因此,数据是极不一致的 [12]。总体而言,大多数专家认为应对患者进行多学科诊疗评估,并且必须进行适当的 RCT 以进一步明确 MPM 的最佳术式[12,22,26,41-43]。 MARS-2 试验目前正在进行中,研究新辅助化疗联合 PD 与单独化疗相比是否能提供生存获益,初步结果应在 2020 年 9 月公布 [26,44,45]。

Full table
Full table
三联模式疗法
很少有 RCT 将多模式治疗与单纯化疗或手术切除进行比较,而将化疗作为三联疗法组成部分的建议基本是根据少数临床试验、可行性试验以及评估无手术机会患者的化疗效果试验中推断出来的。一些学会推荐使用三联疗法(手术、化疗、放疗)[11,20,46,47],尽管英国胸科学会和欧洲呼吸学会建议仅在临床试验时考虑使用多联疗法 [15,48]; 然而,合适的治疗时机仍不确定,如何安排各种治疗手段的次序也无明确依据[48]。此外,即便采用三联疗法,长期生存率也很差,5 年生存率仅有10% [49]。
一般推荐的化疗方案是以铂类为基础的化疗[通常是顺铂,或在老年或较虚弱的患者中使用卡铂[50,51]]加上培美曲塞(叶酸抗代谢药)、B12以及叶酸补充剂[11,15,20,46-48]。添加培美曲塞的建议源于RCTs结果,与单独使用顺铂相比,生存期延长(2.8 个月),同时维生素 B12 和叶酸可降低其毒性 [52,53]。对于身体状况不佳或老年患者,卡铂可提供足够的生存益处,推荐与培美曲塞联合使用 [54]。对于身体状况良好的患者,可以在标准化疗的基础上联合贝伐单抗[血管内皮生长因子 (vascular endothelial growth factor, VEGF) 抑制剂] [11,15,20]。该建议源自一项大型 III 期临床试验MAPS,该试验将单独使用培美曲塞/顺铂与培美曲塞/顺铂联合贝伐单抗进行比较 [55]。该研究表明,联合 VEGF 抑制剂可提高总生存期(提高 2.7 个月);然而,该研究仅在不可切除的 MPM 中进行 [55]。二线化疗一般无标准方案可推荐,建议患者参加临床试验或进行姑息治疗 [11,15,46,48]。美国临床肿瘤学会 (American Society of Clinical Oncology, ASCO) 和国家综合癌症网络 (National Comprehensive Cancer Network, NCCN) 建议对无法参加试验的患者可能使用长春瑞滨作为二线治疗 [11,20],NCCN 还建议免疫治疗和再挑战化疗作为二线治疗 [20]。在一项III 期试验中,与最佳支持性治疗相比,二线培美曲塞治疗在肿瘤客观反应率和延迟疾病进展方面显示出一定效果 [56]。对长春瑞滨源的推荐源于一项 RCT,该试验表明,与积极对症治疗相比,接受长春瑞滨治疗的患者的生存率有升高的趋势(无统计学意义)[57]。其他正在研究的治疗包括 PD 术中胸腔热灌注化疗,尽管除了临床试验外并没有任何学会推荐这种治疗,在推荐这种治疗方案之前需要更多的证据 [58-66]。
大多数评估三联疗法的研究要么是回顾性研究,要么是没有设置对照组或随机条件的可行性研究,仅与历史数据作比较 [67-73]。 2007 年,发表了一项多中心试验,评估在所有患者中采用新辅助化疗(顺铂/吉西他滨 3 个周期)后进行EPP和推荐辅助放疗的可行性[74]。可手术率为 74%,可切除率为 61%,意向性治疗患者的中位总生存期为 19.8 个月,而进行 EPP 的患者为 23 个月[74]。同年,一个意大利小组发表了一项类似的研究,得出了类似的结果[75]。 2009 年,一项多中心 II 期试验结果发布,该试验评估了在三联疗法中新辅助顺铂/培美曲塞的效果 [76]。 I-III 期 MPM 患者先接受4个周期顺铂/培美曲塞治疗,然后没有疾病进展的患者再进行EPP,随后再进行半胸腔辅助放疗 [76]。完成所有治疗的患者中位生存期为 29.1 个月,2 年生存率为 61.2%,其中5%的患者达到研究的主要终点——完全病理缓解;然而,通过意向治疗分析,中位生存期仅为 16.8 个月,2 年生存率为 37.2% [76]。该研究使作者得出结论:在高度选择的患者中,三联疗法可能是有益的 [76]。欧洲癌症研究与治疗组织 (European Organisation for Research and Treatment of Cancer, EORTC) 于 2010 年发表了一项类似的多中心 II 期研究,主要终点定义为“治疗成功”[77]。74% 的患者接受了 EPP,65% 的患者完成了辅助放疗,但只有 42% 的患者满足“治疗成功”的定义,因此作者得出结论:“对于间皮瘤患者,三联疗法虽然可行,但并不能严格在研究规定的时间内完成,因此一定的调整是必要的”[77]。总体而言,尽管在某些患者中可行,但涉及新辅助化疗、EPP 和辅助放疗在内的三联疗法已证明对患者具有相当的挑战性,完成率低,而且对N2期(对侧淋巴结转移,见表1)或病理表现为双相/肉瘤样类型患者的疗效不佳[70,74]。
随着手术方式从EPP转为PD,很少有评估PD三联疗法的研究发表。 2012 年,发表了一项研究,尝试比较三联疗法进行EPP或PD的优劣 [8]。这是一项非随机、前瞻性试验,比较新辅助化疗(顺铂/吉西他滨或顺铂/培美曲塞 3 个周期)后进行 EPP 及辅助放疗与 PD联合胸腔内聚维酮碘热灌注、辅助化疗(4-6 个周期的顺铂/吉西他滨或顺铂/培美曲塞)及预防性放疗[8]。EPP组中,68%的患者完成了所有治疗,其中2名患者存活超过 50 个月(中位存活 12.8 个月)[8];PD组中,大多数患者 (96.3%) 完成了所有治疗,中位生存期为23个月。因此,作者得出结论,在三联模式治疗中,PD 相比EPP更为可行且结局更好[8]。
特别是关于联合辅助或新辅助放疗的相关资料,数据同样是混合型、没有进行对照和随机化控制。如果EPP术后不需要考虑肺部相关的并发症,经典的半胸腔放疗涉及对整个胸膜腔(包括同侧淋巴结床),使用光子/电子辐射进行前后/后前对穿放疗,放疗时要遮挡重要器官[78]。然而,要使其他重要器官(食道、心脏、肠等)免于辐射仍然很困难,并且难以定位后膈角 [79]。调强适形放疗(Intensity-modulated radiation therapy,IMRT) 最早用于头颈部癌和前列腺癌,用计算机进行三维规划使照射剂量的分布更符合靶区特征 [80,81]。 2007 年,一项前瞻性研究评估了 EPP(化疗或未化疗) 术后辅助 IMRT 的局部控制效果,研究显示中位生存期和 3 年生存率有所提高,只有 5% 接受放疗的患者出现了照射野内的局部复发[79]。 2013 年,一项回顾性研究也证明了 EPP 术后辅助 IMRT 的安全性及改善局部复发的作用[82],另一项 II 期临床试验进一步证明了其可行性[83]。2014 年,一个欧洲小组尝试进行一项国际范围内的多中心RCT (SAKK 17/04),以比较联合辅助 IMRT 与单纯新辅助化疗(顺铂/培美曲塞)+EPP的效果 [84]。不幸的是,研究进展缓慢且无法达到 1 年无复发生存的主要终点,导致作者反对常规进行半胸腔辅助放疗 [84]。2014 年的 SMART 试验旨在评估 T1-3N0期MPM 患者进行新辅助 IMRT 继以 EPP 和辅助化疗(顺铂和抗叶酸)后,新辅助IMRT 对ypN2 患者的作用 [同侧或隆突下淋巴结受累,使用第 7 版国际肺癌研究协会 (IASLC) TNM 分级] [5,85]。上皮亚型的 3 年生存率为 84%,而双相亚型为 13%;然而,没有与辅助放疗进行比较 [85]。同年发表的另一项研究评估了 PD 术后的辅助放疗,大多数患者 (95%) 接受了辅助、新辅助或“三明治”化疗 [86]。该研究显示其中位总生存期为 33 个月,PFS 为 29 个月 [86]。鉴于需要密切监测和充分筛选患者,这种疗法基本上不被广泛推荐,除非是在高水平专业医疗中心 [78]。 2016 年,凯特琳癌症研究中心 (Memorial Sloan Kettering Cancer Center, MSKCC) 的一个研究小组公布了 IMPRINT II 期临床试验结果,证明了新辅助化疗+ PD 联合术后辅助 IMRT疗法的可行性以及可以接受的并发症发生率 [87];第二年,同一个研究小组回顾性分析 PD 三联疗法,比较了在该疗法中使用常规放疗和半胸腔 IMRT,发现进行IMRT 的患者总生存期更高(20.2 个月vs 12.3 个月)[88]。总体而言,无论患者进行EPP还是PD,能完成辅助 IMRT 的比例仍然很低,约为三分之二[89]。
至少在美国,ASCO 和 NCCN 指南都建议采用多模式联合治疗,但这种建议似乎没有发挥多大作用,高达 29% 的 I-III 期上皮样 MPM 患者未接受治愈性治疗,尤其是在病例数有限的情况下[90]。对国家癌症数据库的分析进一步表明,只有 20% 的患者接受了针对肿瘤的手术,其中 2.6% 接受了三联疗法 [91],这可能是由于缺乏令人信服的数据,也说明需要进一步开展高质量RCTs [44]。系统评价表明 RCTs 未能完成招募计划,需要包括对照组在内的 II 期临床试验,并需要系统性地发布意向性治疗分析 [92,93]。 2020 年 9 月即将发布的 MARS-2 结果(参见“手术”部分)可能有助于阐明多联疗法的优势 [45]。此外,MSKCC 小组应该在 2021 年 7 月发布他们的 II 期研究结果,该研究比较了PD 联合辅助 IMRT基础上用铂类/培美曲塞进行辅助化疗与新辅助化疗的毒性差异;这可能有助于阐明化疗在三联疗法中的最佳排序 [94]。
新疗法及展望
目前恶性间皮瘤的治疗手段包括手术、化疗和放疗。 然而,尽管采用多种手段治疗,预后仍然很差,诊断后总生存期为9 至17个月[75,95,96]。 鉴于此,许多新出现的、试验性治疗方法可用于对常规治疗无反应的患者,包括免疫治疗、T 细胞疗法、抗肿瘤疫苗和病毒疗法。
免疫治疗
免疫系统在 MPM 中发挥重要作用,肿瘤和免疫系统之间的相互作用主要由局部免疫调节机制驱动,有证据表明针对肿瘤的免疫疗法有全身反应 [97,98]。 当肿瘤被细胞毒性 CD8+ T 细胞(肿瘤浸润性淋巴细胞)高度浸润时,MPM 患者的生存率提高,而程序性死亡配体 1 (programmed death-ligand 1,PDL-1) 的表达与生存率降低有关(PDL-1 阳性vs. 阴性患者中位OS:5个月vs. 14.5 个月;P<0.0001) [99-101]。超说明书使用抗PD -1抗体pembrolizumab或nivolumab作单药治疗,或nivolumab联合细胞毒性T淋巴细胞抗原4 (cytotoxic T lymphocyte antigen 4, CTLA-4)抗体ipilimumab治疗MPM均显示出良好的活性 [102,103]。
Popat等人公布了一项III期试验的初步结果,在该试验中,144例经预处理的晚期MPM患者随机接受派姆单抗或标准化疗。相对于吉西他滨或长春瑞滨组(6%),派姆单抗组的客观缓解率改善了22%。然而,两组的PFS (2.5 vs. 3.4月)和总生存期(10.7 vs. 11.7月)相似[102]。在另一项试验中,Alley等人用派姆单抗治疗了25例PDL -1阳性MPM患者。5例(20%)患者部分缓解,13例(52%)患者病情稳定[104]。
对其他免疫疗法也进行了评估。例如,在法国 21 家医院开展的一项多中心随机、非比较、非盲的2 期临床试验 (MAPS2),旨在评估单用nivolumab与联用ipilimumab的12 周疾病控制情况,结果分别有24 例 (44%,24/54,单药组)和27例(50%,27/54,双药联合组)患者的疾病获得控制。两组的客观缓解率分别为19%(10/54,单药组)和28%(15/54,双药联合组) [103]。另一项单臂 II 期临床试验 (INITIATE) 评估了ipilimumab和nivolumab联合治疗复发性 MPM 的情况。在该研究中,对 34 名患者在 12 周时的治疗反应进行了评估。10例(29%)患者部分缓解,13例(38%)患者病情稳定。然而,33例(94%)患者报告了不良事件,12例(34%)患者报告了3级毒性反应[105]。这些发现显示了单药和双药阻断在MPM中都有很好的作用。
在一项纳入571例患者的II 期随机临床试验 (DETERMINE) 中发现,另一种 CTLA-4 抑制剂 tremelimumab与安慰剂相比并未提供生存获益(中位生存期分别为 7.7 个月和 7.3 个月)[106]。 在 II 期研究中与抗 PDL-1 抗体 durvalumab 联合使用时,40 例患者中有11例 (28%) 显示出免疫相关的客观缓解(均为部分缓解),中位客观缓解持续时间为16.1个月,25例(63%)患者获得疾病控制,中位 PFS 为 5.7 个月,中位总生存期为 16.6个月 [107]。 但是,随机研究的数据是必需的。
使用干扰素或白细胞介素 2 的传统免疫疗法,无论是单独用药还是联合化疗,都没有表现出实质性优势 [108-110]。
T细胞治疗
间皮素 (mesothelin, MSLN) 是一种最近发现的细胞表面糖蛋白和生物标志物,表达于正常的间皮细胞上[111],也表达于恶性间皮瘤和其他实体肿瘤的细胞表面[5]。它已被发现从恶性间皮瘤细胞和某些其他实体瘤中脱落 [112],并且在高达 80%~84% 的 MPM 患者中,MSLN 的血清水平会升高 [113,114]。T细胞治疗是一种很有前景的MPM治疗新策略。一项I 期临床试验旨在研究针对 MPM 患者 MSLN 的嵌合抗原受体 (chimeric antigen receptor, CAR) T 细胞疗法,最近报道的结果令人鼓舞。在这项研究中,18 名患者接受了单剂量的 CD28 共刺激 MSLN CAR-T 细胞和 I-caspase-9 安全基因,胸膜内给药,使用或不使用环磷酰胺预处理。14例患者随后接受了不在试验协议中的抗 PD-1 治疗。这 14例患者经PET检查显示:2例完全缓解(高代谢消失),5例部分缓解,4例病情稳定。关于安全性,未观察到CAR-T细胞治疗相关的超1级的毒性反应[115]。这显示出良好的发展前景,特别是当 CAR-T 细胞疗法与抗 PD-1 疗法相结合时,因为之前的临床前数据表明,CAR-T细胞在肿瘤高负荷的情况下会功能衰竭;而对于某些患者, CAR-T细胞会在PD-1阻断后扩增[116]。
疫苗
另一种激发免疫系统获得性抗肿瘤活性的方法是接种疫苗,这引起了对 MPM 疫苗治疗的重要研究。MPM 中的 Wilms 肿瘤-1 (Wilms tumor-1,WT1) 蛋白是一个有希望的靶点,与正常细胞相比,它在MPM的高度表达[117]使其成为选择性抗肿瘤疫苗的理想靶点。一项双盲、对照的双中心 II 期临床试验将经过预处理的41名MPM 患者随机分配至galinpepimut-S(一种 WT1类似物肽疫苗)+粒细胞-巨噬细胞集落刺激因子 (Granulocyte-macrophage colony-stimulating factor, GM-CSF) + Montanide组,或GM-CSF + Montanide组,GM-CSF 和 Montanide都是免疫佐剂。疫苗组(45%)获得1年PFS较对照组 (33%)比例高,中位PFS 分别为10.1个月和7.4个月;中位OS分别为22.8个月和18.3个月。然而,该研究没有做治疗组之间的比较[118]。鉴于 MPM 的治疗选择有限,这显示了新疗法的前景。
另一个治疗靶点是树突状细胞 (dendritic cell, DC) ,这是一种基于细胞的疫苗疗法,用于激发抗肿瘤免疫反应。DC 将肿瘤相关抗原 (tumor-associated antigens, TAAs) 呈递给淋巴结中的 T 细胞,诱导肿瘤特异性的CD4+和CD8+ T 细胞增殖和激活。癌症患者的 DC 功能受损 [119,120]。在 DC 治疗中,DCs 在体外成熟并激活,以增强免疫原性、避免肿瘤免疫抑制,然后再负载 TAAs。I 期试验显示了其有前景的临床反应和持久的放射学反应[121,122]。最近发起了一项多中心、随机的 II/III 期临床试验,旨在评估化疗后 MPM 患者中负载同种异体肿瘤细胞裂解液(MesoPher)的DCs(DENIM试验),以评估其是否会提高生存率并证明其作为维持治疗的有效性[123]。
病毒疗法
最近溶瘤病毒疗法成为一种颇具前景的实验模式。病毒载体用于感染肿瘤细胞,进而导致肿瘤细胞裂解,再释放肿瘤相关抗原和病毒抗原,从而引发抗肿瘤免疫反应 [124,125]。在大多数病毒载体中,首选的给药途径是瘤内 (intratumoral, IT)给药,鉴于MPM源于胸膜腔及其生长模式,这使其成为研究溶瘤病毒疗法的理想模型 [126,127]。溶瘤病毒疗法临床前和临床试验中研究最多的载体是腺病毒。它在动物实验中显示出肿瘤消退和改善生存的良好结果 [128]。使用腺病毒载体的人体试验显示出良好的安全性,但反应率较低 [129-131]。
临床前试验中研究的另一种病毒是单纯疱疹病毒 1 型 (Herpes simplex virus type 1, HSV-1),但尚无公开的人体试验。一项正在进行的I/IIa期试验是通过单次和多次给予 HSV1716(一种突变型 HSV-1溶瘤病毒)治疗 MPM,旨在评估其安全性和生物学效应[132]。
其他疗法
其他全身性治疗方式也进行了研究,但目前还没有在临床试验环境之外适用。 例如,一项II 期临床试验显示,在常规化疗中加入尼达尼布 [一种血管内皮生长因子受体 (VEGFR)、血小板衍生生长因子受体 (PDGFR) 和成纤维细胞生长因子受体 (FGFR) 酪氨酸激酶抑制剂],对改善 PFS 可能有益 [133],但这一益处未能在III期试验中再现 [134]。同样,Vorinostat(一种口服组蛋白去乙酰化酶抑制剂)也在早期试验中显示出应用潜力 [135],但在一项包含 661名患者的 III 期试验中,与安慰剂相比,Vorinostat没有展现出显著获益 [136]。
结论
MPM 是一种罕见的肿瘤,主要由石棉暴露引起,潜伏期非常长。患者发病时多为晚期,因此,虽经积极治疗,但仍致命且预后不佳。因此,手术干预仍有争议,通常仅适用于多模式治疗中的早期患者。具体术式也多有争议,尽管最近的手术已经从主张首选但激进的 EPP 转变为更保守但目标仅为R1切除的扩大 PD。辅助或新辅助放疗及化疗也是治疗的主要手段,但各种治疗手段的最佳治疗顺序为何?干预时机为何?目前缺乏依据。总体而言,患者的选择仍然至关重要,尤其是在缺乏共识的情况下。
随着我们对MPM认识的不断深入,其他治疗方案也开始出现并不断发展。在过去几年中,人们对遗传学、免疫生物学、生物标志物和肿瘤微环境进行了研究,由此获得的知识为许多新兴疗法打开了大门。目前,有多项临床试验在探询各种新兴疗法及联合疗法。然而,支持使用这些新治疗方式的证据仍然很少,主要是由于缺乏随机临床试验。
鉴于MPM的罕见性,以及缺乏“一刀切式”治疗模式的成功案例,合作开展精心设计的科学研究是很有必要的,以减缓疾病进展,降低当前疗法的并发症发生率,并改善患者生存。
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review Checklist. Available at http://dx.doi.org/10.21037/ccts-20-112
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ccts-20-112). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lucchini RG, Hashim D, Lambertini L, et al. Evidence From Epidemiology and Health Surveillance. Malignant Pleural Mesothelioma: Elsevier; 2019: 1-13.
- Cugell DW, Kamp DW. Asbestos and the pleura: a review. Chest 2004;125:1103-17. [Crossref] [PubMed]
- Ismail-Khan R, Robinson LA, Williams CC Jr, et al. Malignant pleural mesothelioma: a comprehensive review. Cancer Control 2006;13:255-63. [Crossref] [PubMed]
- Hassan R, Morrow B, Thomas A, et al. Inherited predisposition to malignant mesothelioma and overall survival following platinum chemotherapy. Proc Natl Acad Sci U S A 2019;116:9008-13. [Crossref] [PubMed]
- Mutti L, Peikert T, Robinson BWS, et al. Scientific Advances and New Frontiers in Mesothelioma Therapeutics. J Thorac Oncol 2018;13:1269-83. [Crossref] [PubMed]
- Butchart EG, Ashcroft T, Barnsley W, et al. Pleuropneumonectomy in the management of diffuse malignant mesothelioma of the pleura. Experience with 29 patients. Thorax 1976;31:15-24. [Crossref] [PubMed]
- Sugarbaker DJ, Wolf AS. Surgery for malignant pleural mesothelioma. Expert Rev Respir Med 2010;4:363-72. [Crossref] [PubMed]
- Lang-Lazdunski L, Bille A, Lal R, et al. Pleurectomy/decortication is superior to extrapleural pneumonectomy in the multimodality management of patients with malignant pleural mesothelioma. J Thorac Oncol 2012;7:737-43. [Crossref] [PubMed]
- Chauhan D, Vigneswaran WT. History of Pleural Surgical Treatment. In: Hesdorffer M, Bates-Pappas GE, editors. Caring for Patients with Mesothelioma: Principles and Guidelines. Cham: Springer International Publishing; 2019: 3-11.
- Taioli E, Wolf AS, Camacho-Rivera M, et al. Determinants of Survival in Malignant Pleural Mesothelioma: A Surveillance, Epidemiology, and End Results (SEER) Study of 14,228 Patients. PLoS One 2015;10:e0145039. [Crossref] [PubMed]
- Kindler HL, Ismaila N, Armato SG 3rd, et al. Treatment of Malignant Pleural Mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1343-73. [Crossref] [PubMed]
- Bueno R, Opitz I, Taskforce IM. Surgery in Malignant Pleural Mesothelioma. J Thorac Oncol 2018;13:1638-54. [Crossref] [PubMed]
- Flores RM. Pleurectomy decortication for mesothelioma: The procedure of choice when possible. J Thorac Cardiovasc Surg 2016;151:310-2. [Crossref] [PubMed]
- Sugarbaker DJ. Macroscopic complete resection: the goal of primary surgery in multimodality therapy for pleural mesothelioma. J Thorac Oncol 2006;1:175-6. [Crossref] [PubMed]
- Woolhouse I, Bishop L, Darlison L, et al. BTS guideline for the investigation and management of malignant pleural mesothelioma. BMJ Open Respir Res 2018;5:e000266. [Crossref] [PubMed]
- Stahel RA, Weder W, Lievens Y, et al. Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21:v126-8. [Crossref] [PubMed]
- Rusch V, Baldini EH, Bueno R, et al. The role of surgical cytoreduction in the treatment of malignant pleural mesothelioma: meeting summary of the International Mesothelioma Interest Group Congress, September 11-14, 2012, Boston, Mass. J Thorac Cardiovasc Surg 2013;145:909-10. [Crossref] [PubMed]
- Klikovits T, Hoda MA, Dong Y, et al. Management of malignant pleural mesothelioma - part 3: Data from the Austrian Mesothelioma Interest Group (AMIG) database. Wien Klin Wochenschr 2016;128:627-34. [Crossref] [PubMed]
- Pinto C, Novello S, Torri V, et al. Second Italian Consensus Conference on Malignant Pleural Mesothelioma: State of the art and recommendations. Cancer Treat Rev 2013;39:328-39. [Crossref] [PubMed]
- NCCN. NCCN Clinical Practice Guidelines in Oncology - Malignang Pleural Mesothelioma: National Comprehensive Cancer Network; 2019 [updated November 27, 2019]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/mpm.pdf
- Treasure T, Fiorentino F, Utley M, et al. A survey of opinions and beliefs concerning surgery for malignant pleural mesothelioma amongst 802 members of the european association for cardio-thoracic surgery (EACTS), the European society of thoracic surgeons (ESTS) and the society of thoracic surgeons (STS). Interact Cardiovasc Thorac Surg 2011;12:341-6. [Crossref] [PubMed]
- Treasure T. What is the best approach for surgery of malignant pleural mesothelioma? It is to put our efforts into obtaining trustworthy evidence for practice. J Thorac Cardiovasc Surg 2016;151:307-9. [Crossref] [PubMed]
- Utley M, Fiorentino F, Treasure T. Obtaining an upper estimate of the survival benefit associated with surgery for mesothelioma. Eur J Cardiothorac Surg 2010;38:241-4. [Crossref] [PubMed]
- Verma V, Wegner RE, Ludmir EB, et al. Management of Malignant Pleural Mesothelioma in the Elderly Population. Ann Surg Oncol 2019;26:2357-66. [Crossref] [PubMed]
- Takuwa T, Hasegawa S. Current surgical strategies for malignant pleural mesothelioma. Surg Today 2016;46:887-94. [Crossref] [PubMed]
- Datta A, Smith R, Fiorentino F, et al. Surgery in the treatment of malignant pleural mesothelioma: recruitment into trials should be the default position. Thorax 2014;69:194-7. [Crossref] [PubMed]
- Hasegawa S. Extrapleural pneumonectomy or pleurectomy/decortication for malignant pleural mesothelioma. Gen Thorac Cardiovasc Surg 2014;62:516-21. [Crossref] [PubMed]
- Berzenji L, Van Schil PE, Carp L. The eighth TNM classification for malignant pleural mesothelioma. Transl Lung Cancer Res 2018;7:543-9. [Crossref] [PubMed]
- Rusch VW, Giroux D, Kennedy C, et al. Initial Analysis of the International Association For the Study of Lung Cancer Mesothelioma Database. J Thorac Oncol 2012;7:1631-9. [Crossref] [PubMed]
- Spaggiari L, Marulli G, Bovolato P, et al. Extrapleural pneumonectomy for malignant mesothelioma: an Italian multicenter retrospective study. Ann Thorac Surg 2014;97:1859-65. [Crossref] [PubMed]
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620-6. [Crossref] [PubMed]
- Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72. [Crossref] [PubMed]
- Batirel HF, Metintas M, Caglar HB, et al. Adoption of pleurectomy and decortication for malignant mesothelioma leads to similar survival as extrapleural pneumonectomy. J Thorac Cardiovasc Surg 2016;151:478-84. [Crossref] [PubMed]
- Rena O, Casadio C. Extrapleural pneumonectomy for early stage malignant pleural mesothelioma: a harmful procedure. Lung Cancer 2012;77:151-5. [Crossref] [PubMed]
- Cao C, Tian D, Park J, et al. A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer 2014;83:240-5. [Crossref] [PubMed]
- Magouliotis DE, Tasiopoulou VS, Athanassiadi K. Updated meta-analysis of survival after extrapleural pneumonectomy versus pleurectomy/decortication in mesothelioma. Gen Thorac Cardiovasc Surg 2019;67:312-20. [Crossref] [PubMed]
- Vigneswaran WT, Kircheva DY, Rodrigues AE, et al. Influence of Pleurectomy and Decortication in Health-Related Quality of Life Among Patients with Malignant Pleural Mesothelioma. World J Surg 2018;42:1036-45. [Crossref] [PubMed]
- Papaspyros S, Papaspyros S. Surgical Management of Malignant Pleural Mesothelioma: Impact of Surgery on Survival and Quality of Life—Relation to Chemotherapy, Radiotherapy, and Alternative Therapies. ISRN Surg 2014;2014:817203. [Crossref] [PubMed]
- Kostron A, Friess M, Inci I, et al. Propensity matched comparison of extrapleural pneumonectomy and pleurectomy/decortication for mesothelioma patients. Interact Cardiovasc Thorac Surg 2017;24:740-6. [Crossref] [PubMed]
- Sharkey AJ, Tenconi S, Nakas A, et al. The effects of an intentional transition from extrapleural pneumonectomy to extended pleurectomy/decortication. Eur J Cardiothorac Surg 2016;49:1632-41. [Crossref] [PubMed]
- Treasure T, Sedrakyan A. Pleural mesothelioma: little evidence, still time to do trials. Lancet 2004;364:1183-5. [Crossref] [PubMed]
- Fournel L, Janet-Vendroux A, Canny-Hamelin E, et al. Malignant pleural mesothelioma: The role of surgery. Rev Pneumol Clin 2018;74:351-8. [Crossref] [PubMed]
- Treasure T, Macbeth F. Fifteen years in the evaluation of extrapleural pneumonectomy: Lessons to be learned. J Thorac Cardiovasc Surg 2015;149:1382-3. [Crossref] [PubMed]
- Hiddinga BI, van Meerbeeck JP. Surgery in mesothelioma--where do we go after MARS? J Thorac Oncol 2013;8:525-9. [Crossref] [PubMed]
- ClinicalTrials.gov. Mesothelioma and Radical Surgery 2 (MARS2) 2019 [updated October 20, 2019]. Available online: https://clinicaltrials.gov/ct2/show/NCT02040272
- Baas P, Fennell D, Kerr KM, et al. Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v31. [Crossref] [PubMed]
- van Zandwijk N, Clarke C, Henderson D, et al. Guidelines for the diagnosis and treatment of malignant pleural mesothelioma. J Thorac Dis 2013;5:E254. [PubMed]
- Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010;35:479-95. [Crossref] [PubMed]
- Fahrner R, Ochsenbein A, Schmid RA, et al. Long term survival after trimodal therapy in malignant pleural mesothelioma. Swiss Med Wkly 2012;142:w13686. [Crossref] [PubMed]
- Ceresoli GL, Castagneto B, Zucali PA, et al. Pemetrexed plus carboplatin in elderly patients with malignant pleural mesothelioma: combined analysis of two phase II trials. Br J Cancer 2008;99:51-6. [Crossref] [PubMed]
- Katirtzoglou N, Gkiozos I, Makrilia N, et al. Carboplatin Plus Pemetrexed as First-line Treatment of Patients With Malignant Pleural Mesothelioma: A Phase II Study. Clin Lung Cancer 2010;11:30-5. [Crossref] [PubMed]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III Study of Pemetrexed in Combination With Cisplatin Versus Cisplatin Alone in Patients With Malignant Pleural Mesothelioma. J Clin Oncol 2003;21:2636-44. [Crossref] [PubMed]
- Scagliotti GV, Shin DM, Kindler HL, et al. Phase II study of pemetrexed with and without folic acid and vitamin B12 as front-line therapy in malignant pleural mesothelioma. J Clin Oncol 2003;21:1556-61. [Crossref] [PubMed]
- Ceresoli GL, Zucali PA, Favaretto AG, et al. Phase II Study of Pemetrexed Plus Carboplatin in Malignant Pleural Mesothelioma. J Clin Oncol 2006;24:1443-8. [Crossref] [PubMed]
- Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016;387:1405-14. [Crossref] [PubMed]
- Jassem J, Ramlau R, Santoro A, et al. Phase III trial of pemetrexed plus best supportive care compared with best supportive care in previously treated patients with advanced malignant pleural mesothelioma. J Clin Oncol 2008;26:1698-704. [Crossref] [PubMed]
- Muers MF, Stephens RJ, Fisher P, et al. Active symptom control with or without chemotherapy in the treatment of patients with malignant pleural mesothelioma (MS01): a multicentre randomised trial. Lancet 2008;371:1685-94. [Crossref] [PubMed]
- Richards WG, Zellos L, Bueno R, et al. Phase I to II study of pleurectomy/decortication and intraoperative intracavitary hyperthermic cisplatin lavage for mesothelioma. J Clin Oncol 2006;24:1561-7. [Crossref] [PubMed]
- Gomez D, Tsao AS. Local and systemic therapies for malignant pleural mesothelioma. Curr Treat Options Oncol 2014;15:683-99. [Crossref] [PubMed]
- Burt BM, Richards WG, Lee HS, et al. A Phase I Trial of Surgical Resection and Intraoperative Hyperthermic Cisplatin and Gemcitabine for Pleural Mesothelioma. J Thorac Oncol 2018;13:1400-9. [Crossref] [PubMed]
- Bertoglio P, Aprile V, Ambrogi MC, et al. The role of intracavitary therapies in the treatment of malignant pleural mesothelioma. J Thorac Dis 2018;10:S293-7. [Crossref] [PubMed]
- Opitz I, Lauk O, Meerang M, et al. Intracavitary cisplatin-fibrin chemotherapy after surgery for malignant pleural mesothelioma: A phase I trial. J Thorac Cardiovasc Surg 2019. Epub ahead of print. [Crossref] [PubMed]
- Okabe K. Intraoperative intracavitary hyperthermic chemotherapy for malignant pleural mesothelioma. Ann Transl Med 2017;5:233. [Crossref] [PubMed]
- Ried M, Potzger T, Braune N, et al. Local and systemic exposure of cisplatin during hyperthermic intrathoracic chemotherapy perfusion after pleurectomy and decortication for treatment of pleural malignancies. J Surg Oncol 2013;107:735-40. [Crossref] [PubMed]
- Ishibashi H, Kobayashi M, Takasaki C, et al. Interim results of pleurectomy/decortication and intraoperative intrapleural hyperthermic cisplatin perfusion for patients with malignant pleural mesothelioma intolerable to extrapleural pneumonectomy. Gen Thorac Cardiovasc Surg 2015;63:395-400. [Crossref] [PubMed]
- Sugarbaker DJ, Gill RR, Yeap BY, et al. Hyperthermic intraoperative pleural cisplatin chemotherapy extends interval to recurrence and survival among low-risk patients with malignant pleural mesothelioma undergoing surgical macroscopic complete resection. J Thorac Cardiovasc Surg 2013;145:955-63. [Crossref] [PubMed]
- Rusch VW, Rosenzweig K, Venkatraman E, et al. A phase II trial of surgical resection and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2001;122:788-95. [Crossref] [PubMed]
- Van Schil PE, Opitz I, Weder W, et al. Multimodal management of malignant pleural mesothelioma: where are we today? Eur Respir J 2014;44:754-64. [Crossref] [PubMed]
- Buduhan G, Menon S, Aye R, et al. Trimodality Therapy for Malignant Pleural Mesothelioma. Ann Thorac Surg 2009;88:870-5. [Crossref] [PubMed]
- de Perrot M, Feld R, Cho BC, et al. Trimodality therapy with induction chemotherapy followed by extrapleural pneumonectomy and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:1413-8. [Crossref] [PubMed]
- Kapeles M, Gensheimer MF, Mart DA, et al. Trimodality Treatment of Malignant Pleural Mesothelioma: An Institutional Review. Am J Clin Oncol 2018;41:30-5. [PubMed]
- Thieke C, Nicolay NH, Sterzing F, et al. Long-term results in malignant pleural mesothelioma treated with neoadjuvant chemotherapy, extrapleural pneumonectomy and intensity-modulated radiotherapy. Radiat Oncol 2015;10:267. [Crossref] [PubMed]
- Mencoboni M, Filiberti RA, Taveggia P, et al. Clinical Features and Treatment Outcome of Malignant Pleural Mesothelioma. Oncol Res Treat 2017;40:364-9. [Crossref] [PubMed]
- Weder W, Stahel RA, Bernhard J, et al. Multicenter trial of neo-adjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. Ann Oncol 2007;18:1196-202. [Crossref] [PubMed]
- Rea F, Marulli G, Bortolotti L, et al. Induction chemotherapy, extrapleural pneumonectomy (EPP) and adjuvant hemi-thoracic radiation in malignant pleural mesothelioma (MPM): Feasibility and results. Lung Cancer 2007;57:89-95. [Crossref] [PubMed]
- Krug LM, Pass HI, Rusch VW, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:3007-13. [Crossref] [PubMed]
- Van Schil PE, Baas P, Gaafar R, et al. Trimodality therapy for malignant pleural mesothelioma: results from an EORTC phase II multicentre trial. Eur Respir J 2010;36:1362-9. [Crossref] [PubMed]
- Gomez DR, Rimner A, Simone CB 2nd, et al. The Use of Radiation Therapy for the Treatment of Malignant Pleural Mesothelioma: Expert Opinion from the National Cancer Institute Thoracic Malignancy Steering Committee, International Association for the Study of Lung Cancer, and Mesothelioma Applied Research Foundation. J Thorac Oncol 2019;14:1172-83. [Crossref] [PubMed]
- Rice DC, Stevens CW, Correa AM, et al. Outcomes after extrapleural pneumonectomy and intensity-modulated radiation therapy for malignant pleural mesothelioma. Ann Thorac Surg 2007;84:1685-92; discussion 1692-3. [Crossref] [PubMed]
- Ahamad A, Stevens CW, Smythe WR, et al. Intensity-modulated radiation therapy: a novel approach to the management of malignant pleural mesothelioma. Int J Radiat Oncol Biol Phys 2003;55:768-75. [Crossref] [PubMed]
- Taylor A, Powell M. Intensity-modulated radiotherapy—what is it? Cancer Imaging 2004;4:68. [Crossref] [PubMed]
- Gomez DR, Hong DS, Allen PK, et al. Patterns of Failure, Toxicity, and Survival after Extrapleural Pneumonectomy and Hemithoracic Intensity-Modulated Radiation Therapy for Malignant Pleural Mesothelioma. J Thorac Oncol 2013;8:238-45. [Crossref] [PubMed]
- Federico R, Adolfo F, Giuseppe M, et al. Phase II trial of neoadjuvant pemetrexed plus cisplatin followed by surgery and radiation in the treatment of pleural mesothelioma. BMC Cancer 2013;13:22. [Crossref] [PubMed]
- Stahel RA, Riesterer O, Xyrafas A, et al. Neoadjuvant chemotherapy and extrapleural pneumonectomy of malignant pleural mesothelioma with or without hemithoracic radiotherapy (SAKK 17/04): a randomised, international, multicentre phase 2 trial. Lancet Oncol 2015;16:1651-8. [Crossref] [PubMed]
- Cho BC, Feld R, Leighl N, et al. A feasibility study evaluating Surgery for Mesothelioma After Radiation Therapy: the “SMART” approach for resectable malignant pleural mesothelioma. J Thorac Oncol 2014;9:397-402. [Crossref] [PubMed]
- Minatel E, Trovo M, Polesel J, et al. Radical pleurectomy/decortication followed by high dose of radiation therapy for malignant pleural mesothelioma. Final results with long-term follow-up. Lung Cancer 2014;83:78-82. [Crossref] [PubMed]
- Rimner A, Zauderer MG, Gomez DR, et al. Phase II study of hemithoracic intensity-modulated pleural radiation therapy (IMPRINT) as part of lung-sparing multimodality therapy in patients with malignant pleural mesothelioma. J Clin Oncol 2016;34:2761. [Crossref] [PubMed]
- Shaikh F, Zauderer MG, von Reibnitz D, et al. Improved Outcomes with Modern Lung-Sparing Trimodality Therapy in Patients with Malignant Pleural Mesothelioma. J Thorac Oncol 2017;12:993-1000. [Crossref] [PubMed]
- Nelson DB, Rice DC, Mitchell KG, et al. Return to intended oncologic treatment after surgery for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2019;158:924-9. [Crossref] [PubMed]
- Espinoza-Mercado F, Borgella JD, Berz D, et al. Disparities in Compliance With National Guidelines for the Treatment of Malignant Pleural Mesothelioma. Ann Thorac Surg 2019;108:889-96. [Crossref] [PubMed]
- Nelson DB, Rice DC, Niu J, et al. Predictors of trimodality therapy and trends in therapy for malignant pleural mesothelioma. Eur J Cardiothorac Surg 2018;53:960-6. [Crossref] [PubMed]
- De Bondt C, Psallidas I, Van Schil PE, et al. Combined modality treatment in mesothelioma: a systemic literature review with treatment recommendations. Transl Lung Cancer Res 2018;7:562-73. [Crossref] [PubMed]
- Marulli G, Faccioli E, Bellini A, et al. Induction chemotherapy vs post-operative adjuvant therapy for malignant pleural mesothelioma. Expert Rev Respir Med 2017;11:649-60. [Crossref] [PubMed]
- ClinicalTrials.gov. Pleurectomy/Decortication (Neo) Adjuvant Chemothreapy and Intensity Modulated Radiation Therapy to the Pleura in Patients with Locally Advanced Pleural Mesothelioma: Memorial Sloan Kettering Cancer Center; 2008 [updated August 21, 2020]. Available online: https://clinicaltrials.gov/ct2/show/NCT00715611
- Ahamad A, Stevens CW, Smythe WR, et al. Promising early local control of malignant pleural mesothelioma following postoperative intensity modulated radiotherapy (IMRT) to the chest. Cancer J 2003;9:476-84. [Crossref] [PubMed]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [Crossref] [PubMed]
- Sandin LC, Eriksson F, Ellmark P, et al. Local CTLA4 blockade effectively restrains experimental pancreatic adenocarcinoma growth in vivo. Oncoimmunology 2014;3:e27614. [Crossref] [PubMed]
- Fransen MF, van der Sluis TC, Ossendorp F, et al. Controlled local delivery of CTLA-4 blocking antibody induces CD8+ T-cell-dependent tumor eradication and decreases risk of toxic side effects. Clin Cancer Res 2013;19:5381-9. [Crossref] [PubMed]
- Cantini L, Hassan R, Sterman DH, et al. Emerging Treatments for Malignant Pleural Mesothelioma: Where Are We Heading? Front Oncol 2020;10:343. [Crossref] [PubMed]
- Cedrés S, Ponce-Aix S, Pardo-Aranda N, et al. Analysis of expression of PTEN/PI3K pathway and programmed cell death ligand 1 (PD-L1) in malignant pleural mesothelioma (MPM). Lung Cancer 2016;96:1-6. [Crossref] [PubMed]
- Mansfield AS, Roden AC, Peikert T, Sheinin YM, Harrington SM, Krco CJ, et al. B7-H1 expression in malignant pleural mesothelioma is associated with sarcomatoid histology and poor prognosis. J Thorac Oncol 2014;9:1036-40. [Crossref] [PubMed]
- Popat S, Curioni-Fontecedro A, Polydoropoulou V, et al. A multicentre randomized phase III trial comparing pembrolizumab (P) vs single agent chemotherapy (CT) for advanced pre-treated malignant pleural mesothelioma (MPM): Results from the European Thoracic Oncology Platform (ETOP 9-15) PROMISE-meso trial. Ann Oncol 2019;30:v931. [Crossref]
- Scherpereel A, Mazieres J, Greillier L, et al. Nivolumab or nivolumab plus ipilimumab in patients with relapsed malignant pleural mesothelioma (IFCT-1501 MAPS2): a multicentre, open-label, randomised, non-comparative, phase 2 trial. Lancet Oncol 2019;20:239-53. [Crossref] [PubMed]
- Alley EW, Lopez J, Santoro A, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a non-randomised, open-label, phase 1b trial. Lancet Oncol 2017;18:623-30. [Crossref] [PubMed]
- Disselhorst MJ, Quispel-Janssen J, Lalezari F, et al. Ipilimumab and nivolumab in the treatment of recurrent malignant pleural mesothelioma (INITIATE): results of a prospective, single-arm, phase 2 trial. Lancet Respir Med 2019;7:260-70. [Crossref] [PubMed]
- Maio M, Scherpereel A, Calabro L, et al. Tremelimumab as second-line or third-line treatment in relapsed malignant mesothelioma (DETERMINE): a multicentre, international, randomised, double-blind, placebo-controlled phase 2b trial. Lancet Oncol 2017;18:1261-73. [Crossref] [PubMed]
- Calabrò L, Morra A, Giannarelli D, et al. Tremelimumab combined with durvalumab in patients with mesothelioma (NIBIT-MESO-1): an open-label, non-randomised, phase 2 study. Lancet Respir Med 2018;6:451-60. [Crossref] [PubMed]
- Parra HS, Tixi L, Latteri F, et al. Combined regimen of cisplatin, doxorubicin, and alpha-2b interferon in the treatment of advanced malignant pleural mesothelioma: a Phase II multicenter trial of the Italian Group on Rare Tumors (GITR) and the Italian Lung Cancer Task Force (FONICAP). Cancer 2001;92:650-6. [Crossref] [PubMed]
- Halme M, Knuuttila A, Vehmas T, et al. High-dose methotrexate in combination with interferons in the treatment of malignant pleural mesothelioma. Br J Cancer 1999;80:1781-5. [Crossref] [PubMed]
- Bretti S, Berruti A, Dogliotti L, et al. Combined epirubicin and interleukin-2 regimen in the treatment of malignant mesothelioma: a multicenter phase II study of the Italian Group on Rare Tumors. Tumori 1998;84:558-61. [Crossref] [PubMed]
- Pastan I, Hassan R. Discovery of mesothelin and exploiting it as a target for immunotherapy. Cancer Res 2014;74:2907-12. [Crossref] [PubMed]
- Ho M, Onda M, Wang QC, et al. Mesothelin is shed from tumor cells. Cancer Epidemiol Biomarkers Prev 2006;15:1751. [Crossref] [PubMed]
- Hassan R, Remaley AT, Sampson ML, et al. Detection and quantitation of serum mesothelin, a tumor marker for patients with mesothelioma and ovarian cancer. Clin Cancer Res 2006;12:447-53. [Crossref] [PubMed]
- Robinson BW, Creaney J, Lake R, et al. Mesothelin-family proteins and diagnosis of mesothelioma. Lancet 2003;362:1612-6. [Crossref] [PubMed]
- Adusumilli PS, Zauderer MG, Rusch VW, et al. Regional delivery of mesothelin-targeted CAR T cells for pleural cancers: Safety and preliminary efficacy in combination with anti-PD-1 agent. J Clin Oncol 2019;37:2511. [Crossref]
- Chong EA, Svoboda J, Dwivedy Nasta S, et al. Sequential Anti-CD19 Directed Chimeric Antigen Receptor Modified T-Cell Therapy (CART19) and PD-1 Blockade with Pembrolizumab in Patients with Relapsed or Refractory B-Cell Non-Hodgkin Lymphomas. Blood 2018;132:4198. [Crossref]
- Mundlos S, Pelletier J, Darveau A, et al. Nuclear localization of the protein encoded by the Wilms' tumor gene WT1 in embryonic and adult tissues. Development 1993;119:1329-41. [PubMed]
- Zauderer MG, Tsao AS, Dao T, et al. A Randomized Phase II Trial of Adjuvant Galinpepimut-S, WT-1 Analogue Peptide Vaccine, After Multimodality Therapy for Patients with Malignant Pleural Mesothelioma. Clin Cancer Res 2017;23:7483-9. [Crossref] [PubMed]
- Gabrilovich DI, Ciernik IF, Carbone DP. Dendritic cells in antitumor immune responses. I. Defective antigen presentation in tumor-bearing hosts. Cell Immunol 1996;170:101-10. [Crossref] [PubMed]
- Enk AH, Jonuleit H, Saloga J, et al. Dendritic cells as mediators of tumor-induced tolerance in metastatic melanoma. Int J Cancer 1997;73:309-16. [Crossref] [PubMed]
- Hegmans JP, Veltman JD, Lambers ME, et al. Consolidative dendritic cell-based immunotherapy elicits cytotoxicity against malignant mesothelioma. Am J Respir Crit Care Med 2010;181:1383-90. [Crossref] [PubMed]
- Cornelissen R, Hegmans JP, Maat AP, et al. Extended Tumor Control after Dendritic Cell Vaccination with Low-Dose Cyclophosphamide as Adjuvant Treatment in Patients with Malignant Pleural Mesothelioma. Am J Respir Crit Care Med 2016;193:1023-31. [Crossref] [PubMed]
- Belderbos RA, Baas P, Berardi R, et al. A multicenter, randomized, phase II/III study of dendritic cells loaded with allogeneic tumor cell lysate (MesoPher) in subjects with mesothelioma as maintenance therapy after chemotherapy: DENdritic cell Immunotherapy for Mesothelioma (DENIM) trial. Transl Lung Cancer Res 2019;8:280-5. [Crossref] [PubMed]
- Seymour LW, Fisher KD. Oncolytic viruses: finally delivering. Br J Cancer 2016;114:357-61. [Crossref] [PubMed]
- Vachani A, Moon E, Wakeam E, et al. Gene therapy for mesothelioma and lung cancer. Am J Respir Cell Mol Biol 2010;42:385-93. [Crossref] [PubMed]
- Sterman DH. Gene therapy for malignant pleural mesothelioma. Hematol Oncol Clin North Am 2005;19:1147-73. viii. [Crossref] [PubMed]
- Pease DF, Kratzke RA. Oncolytic Viral Therapy for Mesothelioma. Front Oncol 2017;7:179. [Crossref] [PubMed]
- Elshami AA, Kucharczuk JC, Zhang HB, et al. Treatment of pleural mesothelioma in an immunocompetent rat model utilizing adenoviral transfer of the herpes simplex virus thymidine kinase gene. Hum Gene Ther 1996;7:141-8. [Crossref] [PubMed]
- Sterman DH, Treat J, Litzky LA, et al. Adenovirus-mediated herpes simplex virus thymidine kinase/ganciclovir gene therapy in patients with localized malignancy: results of a phase I clinical trial in malignant mesothelioma. Hum Gene Ther 1998;9:1083-92. [Crossref] [PubMed]
- Sterman DH, Molnar-Kimber K, Iyengar T, et al. A pilot study of systemic corticosteroid administration in conjunction with intrapleural adenoviral vector administration in patients with malignant pleural mesothelioma. Cancer Gene Ther 2000;7:1511-8. [Crossref] [PubMed]
- Sterman DH, Recio A, Vachani A, et al. Long-term follow-up of patients with malignant pleural mesothelioma receiving high-dose adenovirus herpes simplex thymidine kinase/ganciclovir suicide gene therapy. Clin Cancer Res 2005;11:7444-53. [Crossref] [PubMed]
- Muggerud AA, Hallett M, Johnsen H, et al. Molecular diversity in ductal carcinoma in situ (DCIS) and early invasive breast cancer. Mol Oncol 2010;4:357-68. [Crossref] [PubMed]
- Grosso F, Steele N, Novello S, et al. Nintedanib Plus Pemetrexed/Cisplatin in Patients With Malignant Pleural Mesothelioma: Phase II Results From the Randomized, Placebo-Controlled LUME-Meso Trial. J Clin Oncol 2017;35:3591-600. [Crossref] [PubMed]
- Scagliotti GV, Gaafar R, Nowak AK, et al. Nintedanib in combination with pemetrexed and cisplatin for chemotherapy-naive patients with advanced malignant pleural mesothelioma (LUME-Meso): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet Respir Med 2019;7:569-80. [Crossref] [PubMed]
- Kelly WK, O'Connor OA, Krug LM, et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol 2005;23:3923-31. [Crossref] [PubMed]
- Krug LM, Kindler HL, Calvert H, et al. Vorinostat in patients with advanced malignant pleural mesothelioma who have progressed on previous chemotherapy (VANTAGE-014): a phase 3, double-blind, randomised, placebo-controlled trial. Lancet Oncol 2015;16:447-56. [Crossref] [PubMed]

张少伟
男,联勤保障部队第九八九医院心胸外科主治医师,解放军医学院外科学硕士,临床工作10余年,擅长胸腔镜下常规肺癌、胸腺瘤及食管癌根治手术,发表论文多篇,其中SCI论著一篇,申请实用新型专利1项。(更新时间:2021/10/8)
(本译文仅供学术交流,实际内容请以英文原文为准。)
Cite this article as: Di Lena É, Aboalsaud A, Sirois C, Mulder D, Spicer J, Ferri L, Cools-Lartigue J. A narrative review of current treatment strategies and emerging therapies in malignant pleural mesothelioma. Curr Chall Thorac Surg 2021;3:8.


