
Implementation of eras for patients undergoing esophagectomy: a narrative review of the current literature and latest evidence
Introduction
The idea of studying sustained recovery after surgery began in 1990 with Krohn et al. (1). They observed that the main causes of rehospitalization and death after cardiac surgery were noncardiac disorders. Even though minimal and preventable, these problems, where neglected, could produce a domino effect on these patients. Conversely, the duration of hospital stay (HS) was not a factor associated to a greater mortality rate when compared to other studies (2). Thereafter, The term “fast-track recovery” appeared for the first time in 1993 as a new protocol applied at Hartford Hospital and the Baystate Medical Center for coronary artery bypass graft surgery (3). According to this report the administration of steroids to limit the inflammatory response, reduced narcotics after surgery, reduced weight gain by reducing fluid administration, prophylactic digoxin to control heart rate, and early ambulation were effective in improving outcome and reducing HS. Lately, the father of the modern concept of enhanced recovery after surgery (ERAS) was undoubtedly Henrik Kehlet, a Danish colorectal surgeon who demonstrated how postoperative complications were not only related to surgical or anesthetic management failure but also influenced by the surgical stress response. He recommended a multimodal preoperative, intraoperative and postoperative strategy based on the co-operation between the healthcare provider and the patient (4). In 2001 a collective of European Surgeons founded an ERAS study group in order to produce evidence-based guidelines for all surgical specialties. This group published their first consensus protocol for patients undergoing colonic surgery in 2005 (5). These efforts led to randomized studies and a subsequent meta-analysis demonstrating that ERAS can reduce postoperative morbidity and HS (6).
No surprisingly, thoracic surgeons have also started to explore the role of ERAS on the surgery of pulmonary resections for lung cancer (7,8). Common elements for ERAS in thoracic surgery include pre-operative optimization with smoking cessation, pre-operative exercise regimes, intra-operative care modifications such as a focus on minimally invasive techniques, chest-drain limitation, and post-operative factors such as early ambulation, early drain removal and long-term narcotic avoidance. Muehling et al. previously reported a randomized study using a fast track approach after lung surgery (9). A significant difference in post-operative pulmonary complications was seen (35% vs. 6.6% in the fast-track group). Pulmonary complications that were reported in this study included atelectasis, pneumonia, prolonged air-leak, pleural effusion, and empyema. Although the incidence of all the specific pulmonary complications were higher in the conservative treatment group, neither group reported any patients with post-operative empyema. This difference in pulmonary complications was more evident in a sub-group of patients with reduced preoperative forced expiratory volume in the first second (FEV1) (55% vs. 7%). Some of us (SF and HCF) have previously reported our experience on 304 patients with ERAS after pulmonary resection (10). A key element in this program was the use of early ambulation. In particular, patients started ambulation within one hour of arrival to the post-anesthesia care unit (PACU), with a target of 250 feet. Initially this target was achieved in only 37.3% of the patients, but afterwards this proportion increased to 72%. However most patients walked, with 68.4% achieving some degree of ambulation within the first hour and 94.7% achieving this within two hours. Overall outcomes were excellent, with a median HS of 1 day, and low pneumonia (0.7%) and atrial fibrillation (4%) rates.
Encouraged by the success of ERAS after pulmonary procedures, surgeons have started to evaluate the role of ERAS after esophagectomy. In the following review we analyze the specific challenges facing patients with esophageal cancer and their care-givers. Similarly to other surgical procedures, ERAS for esophagectomy should be considered using a multimodal approach applied to the various phases of care, namely the pre-operative, intra-operative and post-operative phases. However, challenges and opportunities able to affect changes are perhaps amplified in each of these phases.
Specific challenges related to esophagectomy
Esophageal cancer is a relatively uncommon malignancy. However similar to lung cancer, patients often present at diagnosis with advanced disease-stage, thus implying a poor overall survival. Therefore, early identification and treatment is critical for best outcomes. Patients are often undernourished and this status may conflict with the administration of neoadjuvant therapy. During this critical period there may be a further decline in clinical status, which requires optimization. The operation itself is a complex procedure involving abdominal, thoracic and often cervical components. Traditionally, surgeons place multiple drains including chest, nasogastric and feeding tubes. Different surgical units often vary in extubation timing, use of the intensive care-unit, timing of drain removal, as well as timing and route of enteral nutrition. Due to the procedure duration, the multicavity operative approach and physiologic differences in perfusion, compared to patients undergoing lung resection, patients usually receive more intravenous fluids in the perioperative and post-operative phases. This will lead to more third-spacing of fluids, placing patients at increased risk of pulmonary complications in the post-operative period. Additionally, it is recommended to avoid hypotension in order to minimize ischemia to the gastric conduit used to replace the esophagus. HS is usually longer and therefore requires a greater commitment by staff to ambulation and pulmonary hygiene. Mortality at baseline can be higher than after lung resections. Low et al. reported a 30 and 90-day mortality of 2.4% and 4.5% respectively for high-volume hospitals, reaching a peak of 23% in low-volume hospitals (11,12).
Minimally invasive approaches to esophagectomy can improve the results of the procedure. In a multicenter co-operative group study from the United States 30-day mortality and pneumonia rates were 2.9% and 3.8%, respectively (13). A randomized study from Europe compared minimally invasive to open esophagectomy (14). Pulmonary infections were reported in 12% of the minimally invasive group, which was significantly lower than 34% documented after open esophagectomy. HS was also significantly shorter being 11 vs. 14 days. Although minimally invasive approaches can improve results, there is also significant potential for further improvement in outcomes with adoption of ERAS.
Preoperative phase
Quoting a 2017 editorial from Wynter-Blyth and Moorthy, “Major surgery is like running a marathon—and both require training” (15). Prehabilitation consist of behavioral modification aiming to improve general health and wellbeing prior to major surgery, assuming that preoperative period is a “teachable moment” empowered by a high degree of patient motivation (16,17). Physical inactivity along with poor fitness status negatively affects post-operative outcome (18). In this scenario, 3 weeks of physical exercise prior to surgery seems to improve strength reserve and therefore reducing postoperative complication with a shorter hospital stay (19). Frequency, intensity, time and type (FITT) of exercise should be adjusted based on risk factors and specific needs of the patient (20). Most patients undergoing esophagectomy will be elderly and likely have other co-morbid conditions, that will limit their ability to undergo strenuous exercise. However, education and establishing simple goals such as a modest walking program should be achievable for most patients.
Malnutrition can affect up to 80% of patients with esophageal cancer (21). Nutritional status assessment is the first step in the evaluation of patient undergoing esophagectomy. Evaluation can be performed according to European Society for Clinical Nutrition and Metabolism guidelines (22). In the case of weight loss less than 5%, dietary counseling is sufficient. When weight loss is between 5% and 9%, protein and dietary supplement are useful. Enteral support with tube feeding, is recommended in patients with a weight loss greater than 10%. This factor is the strongest predictor of poor overall survival (23). Adequate nutritional support was effective in decreasing postoperative complications (24,25). This issue is particularly effective for those patients who require neoadjuvant treatment. Indeed, malnutrition increases with the stage of disease; therefore an additional risk is present even before chemo-radiotherapy starts (26). Nutritional status may worsen side effects (e.g., radiation esophagitis, nausea and vomiting) of those treatments and subsequently decrease response rate and ability to tolerate full treatment (27,28).
For these patients, preoperative nutrition may be of paramount importance in order to optimize nutritional status during induction treatment. Although nasoenteral feeding is a simple option, long-term use is uncomfortable and can be associated with aspiration (29). Gastric or jejunal feeding tube placement is the preferred option and can be positioned either using an open, laparoscopic or percutaneous approach. Although some favor the use of a percutaneous gastrostomy tube, our preference is to place a laparoscopic feeding jejunostomy when required. This choice is motivated by the concerns of injuring the stomach, which will usually be used as a conduit to replace the esophagus. Additionally, at the time of laparoscopic jejunostomy laparoscopic staging can also be performed prior to esophagectomy.
Esophageal stenting is another option. Ideally, this should be with a fully covered stent that can be easily removed once induction therapy is completed. However, during induction therapy those stents can migrate, and in some cases are associated with erosion and perforation of the esophagus (30,31). Moreover, extensive inflammation caused by the stent may hinder surgical dissection, especially close to the airways.
As a general rule, patients should be well-educated about ERAS principles. It is possible to achieve proper information through different modalities: verbal explanation, written documents and audio-video materials (32). Anxiety can be a factor for poor outcome with a correlation to prolonged convalescence and postsurgical fatigue (33). In order to reduce fear, anxiety, and overall stress it is important to have good preoperative counseling. On the other hand, we must keep in mind that not all patients want a complete vision of their surgical plan. A proper balance must be undertaken in order to not evoke more fear and anxiety (34). Smoking and alcohol cessation at least 4 weeks before surgery has proven effective in reducing postoperative pneumonia, myocardial ischemia, arrhythmias and nightly hypoxemic episodes (35,36).
Overnight fasting, once considered the standard in patients undergoing elective surgery, has shown to be deleterious as it can result in cardiovascular complications and infections (37,38). Many ERAS programs recommend oral intake of clear fluids, especially those rich in complex carbohydrates, up to 2 hours before surgery as it improves insulin resistance, postoperative nausea and shortens HS (39-41). This recommendation may be not completely feasible in patients undergoing esophagectomy, particularly, in those with bulky, obstructing cancers associated with dysphagia. However, in patients with non-bulky T1bN0, T2N0 cancers and who have no evidence of delayed gastric emptying, it may be reasonable to allow clear fluids intake up to 2 hours before surgery, though this strategy requires further study.
Operative phase
Data from a previous meta-analysis has demonstrated that minimally invasive surgery can reduce post-operative complications and mortality in comparison to traditional open surgery (42). Also, data from the TIME trial has shown a superiority of minimally invasive esophagectomy over open surgery with respect to short-term outcomes, but with similar 3-year overall and disease-free survival (14,43). Lung injury after one lung ventilation can range from mild damage to severe acute respiratory distress syndrome, the latter being associated up to 40% mortality rate (44,45). Protective ventilation strategies, such as low tidal volume, positive end-expiratory pressure, low airway pressure, permissive hypercapnia and sufficient O2 administration to maintain SpO2 greater than 90% reduce the risks of pulmonary complications (46). No difference in oxygenation was found between the use of total intravenous vs. inhalation-based anesthesia during one lung ventilation. However, a retrospective study found a lower overall and disease-free survival in esophageal cancer when inhalational agents were used (47). Same authors demonstrated that propofol had a protective anti-oxidant action and preserved natural-killer cell activity. Nevertheless, randomized trials are necessary to prove any protective or adverse action of anesthetic drugs with respect to cancer-related survival (48).
Fluid management is a challenging issue during esophageal surgery. On one hand, the operation requires extensive dissection within the thoracic and abdominal cavities, and similar to major abdominal surgery, a strategy of liberal fluid management has been applied. Excessive perioperative fluid can cause tissue edema, delayed return of gastrointestinal function, increased pulmonary edema and delayed extubation. On the other hand, a strategy of restrictive fluid management that is often applied in pulmonary surgery, particularly for patients undergoing pneumonectomy, may be associated with hypotension, a need for vasopressors drugs that can lead to ischemia of the gastric conduit, and so this should also be avoided. Taking into account these concerns, the ERAS society recommended a “goal-directed” or “balanced” fluid therapy (49). Their primary recommendation was to avoid a positive fluid balance resulting in a weight gain greater than 2 kg per day. However, intraoperative monitoring of vascular volume status is challenging. Our own preference is to limit epidural catheters in order to minimize hypotension. The use of a minimally invasive surgery facilitates this strategy. Regional anesthesia, such as erector spinae, serratus anterior or intercostal block, is an effective solution for pain control in minimally invasive surgery but none of them has proven a superiority over the others (50). Central venous pressure is occasionally used for fluid monitoring, but it is not accurate in determining cardiac preload, in predicting fluid responsiveness, and in alerting about the onset of pulmonary edema (51,52). On the other hand, the arterial line connected to a Flotrac™ sensor to constantly visualize stroke volume variation and monitor cardiac output has proven effective in this setting (53). This method has been our preference. In our practice central lines are rarely used, and arterial lines will be discontinued at the end of operations soon after extubation unless intensive care unit (ICU) admission is required. Routine placement of abdominal and neck drainages is not necessary and just one chest tube is sufficient.
Postoperative phase
Advantages from routine use of ICU in patients undergoing esophagectomy are questionable. There is a large variability in its usage and no significant impact on outcome has been demonstrated so far. Cerfolio et al. observed that one and half years after introducing an ERAS protocol, avoiding the ICU was safe, less expensive with improved patient satisfaction (54). Interestingly, all operations performed in this report were open Ivor-Lewis esophagectomies. Early extubation has proven to be effective for both cardiac and non-cardiac surgery in reducing the need of ICU admission and favoring management in a Step Down Unit (55-57).
Early ambulation is one of the mainstays of several ERAS protocols, aiming to avoid complications from prolonged bed rest (e.g., pulmonary complications, deep vein thromboses, lean mass loss) (58,59). Ambulation usually starts on post-operative day (POD) 1 or 2, yet it is possible to start walking on the same day of surgery. In our recent paper using a dedicated protocol after video-assisted lobectomy, ambulation started after arrival to the post anesthesia care unit and before moving to the Step Down Unit. In these patients a target of 250 feet within one hour of extubation was set. After arrival to the Step Down Unit, the target rises to 2,500 feet (10 laps) on the day of surgery and 20 laps (5,000 feet) on the following POD that the patient remains in the hospital (60) (Figure 1). We have modified this protocol for patients undergoing esophagectomy. Bearing in mind the longer duration of operation and the presence of more lines/tubes, we still start ambulation in the post anesthesia care unit, depending on staff availability and the time of arrival to the post anesthesia care unit. After transfer to their Step Down Unit bed, ambulation continues with a goal of 5 laps on POD 0, and a minimum of 10 laps/day during the remainder of their hospital stay.
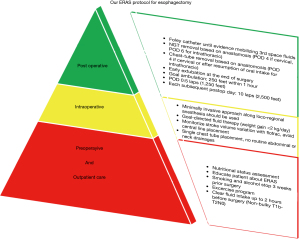
Early removal of urinary catheter should have a positive effect on reducing urinary tract infection rate, but this strategy is debatable after esophagectomy. Indeed, the early removal of the urinary catheter is not indicated in patients with epidural analgesia, due to high risk of urinary retention and subsequent catheter re-insertion (61,62). As mentioned earlier, a “balanced fluid” therapy approach is ideal, and a good assessment of urinary output will help optimize this. For this reason, we routinely keep the urinary catheter in place until the patient demonstrates that they are mobilizing their third space fluids (usually around 72 hours).
Similarly, routine use of nasogastric tube has been questioned as it may decrease postoperative complication rate (63). Arguing against this, a trial comparing nasogastric tube vs. no nasogastric tube showed increased respiratory complications in patients without a nasogastric tube (64). Therefore, use of nasogastric tube is still highly recommended. In another study, early removal (POD2) had no significant increase of pulmonary complications nor anastomotic leaks when compared to late removal (POD6–10) (65). Thus, early removal on POD2 may have some benefits, and will need further and more focused investigation. The location of the anastomosis may influence this issue. In fact, an intrathoracic anastomotic leak can be associated with significant septic issues that may be minimized by a nasogastric tube. Therefore, while we adopt an early removal strategy after a cervical anastomosis, we prefer to leave the nasogastric tube in place until an upper gastrointestinal study has been performed in order to evaluate the optimal healing of the intra-thoracic anastomosis. Cerfolio et al. described an alternative approach after Ivor-Lewis esophagectomy consisting of routine removal of the nasogastric tube on POD 3 followed by an upper gastrointestinal study on POD 4 allowing discharge by POD 7 (54).
It is generally recommended to restrict the number and duration of the chest-tubes. Early removal of chest tube in esophagectomy is safe and not associated with a higher rate of pulmonary complication, as demonstrated by Sato et al. in a recent paper (66). They divided patients into early (POD 1 if <300 mL non-turbid liquid and no air leak) and late removal groups, finding no differences in complication rate. Experience from major lung resection confirms that is possible to safely remove chest tubes even when daily output is up to 450 mL (67). A water-seal system is effective enough and no suction is generally needed, since lung resection has not been performed (68). Again, the timing of removal has to be balanced against the risks of an undrained anastomotic leak. In our practice, we favor early removal when a cervical anastomosis has been performed. Conversely, when an intra-thoracic anastomosis has been performed, chest tube removal is postponed until after resumption of oral feeding.
Enteral nutrition is preferable to total parenteral nutrition due to lower infective complications (69). However, the ideal method to re-introduce enteral nutrition is still under debate. Most surgeons do not reinstate early oral feeding. Nevertheless, a recent randomized study from China compared 140 patients starting liquids on POD1 and then progressing their diet, to a similar control group where oral feeding was started on POD 7 (70). All patients had undergone a minimally invasive esophagectomy with a cervical anastomosis. Notably, there was no difference in complication rates, and the early oral nutrition group demonstrated an early time to first flatus, bowel movement, and superior quality of life scores at two weeks. Most surgeons recommend early enteral nutrition, although the optimal route is unclear (49,71). Our approach is to routinely place a jejunostomy tube. Low-rate jejunal feeding will start on POD2 and then progresses. By the time of discharge, patients will be receiving oral nutrition with supplemental night-time jejunal feeds.
Based on the available literature and evidence, a summary of recommendation is suggested for each phase in the patient care-pathway:
- Preoperative:
- Prehabilitation is the starting point for a good outcome after major surgery and 2–3 weeks of physical exercise prior to operation can improve outcomes. Intensity should be decided according to the general status of patient, but should exceed their baseline activity;
- Assessment of nutritional status is a mainstay, as malnutrition is frequently associated with esophageal cancer. Nutritional supplementation can be decided according to weight loss rate, ranging from simple dietary optimization to enteral nutrition. When necessary, surgical jejunostomy is a good option that can be performed in conjunction with laparoscopic staging;
- Smoking and alcohol cessation 3 weeks prior to operation;
- Patients should be educated about ERAS;
- Clear fluids intake up to 2 hours prior surgery can be allowed in patients with non-bulky T1bN0, T2N0 cancers and no evidence of delayed gastric emptying.
- Intraoperative:
- Utilize a minimally invasive approach when feasible;
- Protective ventilation strategies (e.g., low tidal volume, positive end-expiratory pressure, permissive hypercapnia) to reduce the risks of pulmonary complications;
- “goal-directed” fluid management (can be achieved using an arterial line connected to a Flotrac™ sensor);
- Single chest tube;
- Extubation in the operating room.
- Postoperative:
- Early ambulation starting on the day of operation;
- Set ambulation targets for each post-operative day;
- Maintain urinary catheter until mobilization of third space fluids is achieved, usually after 72 hours;
- Consideration of early removal of a nasogastric tube (particularly for cervical anastomosis);
- Consideration for early removal of chest tube (particularly for cervical anastomosis);
- Consideration for early institution of enteral nutrition (our preference is to start jejunal tube feeds on POD 2).
A focus on recommendations is available at Table 1.
Table 1
| Phase | Recommendations | Ref. |
|---|---|---|
| Preoperative | Assessment of nutritional status is a mainstay. Patients should be stratified and treated according to weight loss rate | Weimann et al. (22) |
| Intraoperative | “Goal-directed” fluid-therapy avoid overload or ipoperfusion during surgical procedure | Low et al. (49) |
| Postoperative | Early extubation is effective in reducing need for ICU | Mandell et al. (56) |
ICU, intensive care unit.
Conclusions
Compared to other surgical areas, ERAS for patients undergoing esophagectomy is still in its infancy. A 2017 meta-analysis demonstrated a reduction of both complications and HS when ERAS was applied (72). Additionally, a guidelines statement for esophagectomy was recently published in 2019 by the ERAS society addressing many of these issues (49). The management of patients undergoing esophagectomy is complex and variable between institutions. Much of the derived post-operative care pathways are ingrained from training, prior experience, and institutional biases. Additionally, different surgeons may employ very different post-operative pathways. The ERAS approach provides an opportunity to improve outcomes. ERAS requires consideration of several components of care from pre-operative to post-operative phases, and developing strategies based on best available evidence that can optimize care. Significant buy-in from all stakeholders is necessary to allow successful evaluation, and ultimately, implementation of best practices.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts-20-105/coif). SK reports personal fees from Medtronic, Auris, Astra Zeneca, and Boston Scientific, outside the submitted work; GV reports honoraria from AbMedica SpA, Medtronic, and Verb Medical, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Krohn BG, Kay JH, Mendez MA, et al. Rapid sustained recovery after cardiac operations. J Thorac Cardiovasc Surg 1990;100:194-7. [Crossref] [PubMed]
- Stanton BA, Jenkins CD, Goldstein RL, et al. Hospital readmissions among survivors six months after myocardial revascularization. JAMA 1985;253:3568-73. [Crossref] [PubMed]
- Cotton P. Fast-track improves CABG outcomes. JAMA 1993;270:2023. [Crossref] [PubMed]
- Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth 1997;78:606-17. [Crossref] [PubMed]
- Fearon KC, Ljungqvist O, Von Meyenfeldt M, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr 2005;24:466-77. [Crossref] [PubMed]
- Greco M, Capretti G, Beretta L, et al. Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg 2014;38:1531-41. [Crossref] [PubMed]
- Semenkovich TR, Hudson JL, Subramanian M, et al. Enhanced Recovery After Surgery (ERAS) in Thoracic Surgery. Semin Thorac Cardiovasc Surg 2018;30:342-9. [Crossref] [PubMed]
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS®) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
- Muehling BM, Halter GL, Schelzig H, et al. Reduction of postoperative pulmonary complications after lung surgery using a fast track clinical pathway. Eur J Cardiothorac Surg 2008;34:174-80. [Crossref] [PubMed]
- Khandhar SJ, Schatz CL, Collins DT, et al. Thoracic enhanced recovery with ambulation after surgery: a 6-year experience. Eur J Cardiothorac Surg 2018;53:1192-8. [Crossref] [PubMed]
- Low DE, Kuppusamy MK, Alderson D, et al. Benchmarking Complications Associated with Esophagectomy. Ann Surg 2019;269:291-8. [Crossref] [PubMed]
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002;346:1128-37. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Franchetti Y, et al. Minimally invasive esophagectomy: results of a prospective phase II multicenter trial-the eastern cooperative oncology group (E2202) study. Ann Surg 2015;261:702-7. [Crossref] [PubMed]
- Straatman J, van der Wielen N, Cuesta MA, et al. Minimally Invasive Versus Open Esophageal Resection: Three-year Follow-up of the Previously Reported Randomized Controlled Trial: the TIME Trial. Ann Surg 2017;266:232-6. [Crossref] [PubMed]
- Wynter-Blyth V, Moorthy K. Prehabilitation: preparing patients for surgery. BMJ 2017;358:j3702. [Crossref] [PubMed]
- Durrand J, Singh SJ, Danjoux G. Prehabilitation. Clin Med (Lond) 2019;19:458-64. [Crossref] [PubMed]
- Flocke SA, Clark E, Antognoli E, et al. Teachable moments for health behavior change and intermediate patient outcomes. Patient Educ Couns 2014;96:43-9. [Crossref] [PubMed]
- Moran J, Wilson F, Guinan E, et al. The preoperative use of field tests of exercise tolerance to predict postoperative outcome in intra-abdominal surgery: a systematic review. J Clin Anesth 2016;35:446-55. [Crossref] [PubMed]
- Valkenet K, van de Port IG, Dronkers JJ, et al. The effects of preoperative exercise therapy on postoperative outcome: a systematic review. Clin Rehabil 2011;25:99-111. [Crossref] [PubMed]
- Macmillan Cancer Support. Principles and guidance for prehabilitation within the management and support of people with cancer. 2019. Available online: https://www.macmillan.org.uk/healthcare-professionals/news-and-resources/guides/principles-and-guidance-for-prehabilitation
- Larrea J, Vega S, Martínez T, et al. The nutritional status and immunological situation of cancer patients. Nutr Hosp 1992;7:178-84. [PubMed]
- Weimann A, Braga M, Carli F, et al. ESPEN guideline: Clinical nutrition in surgery. Clin Nutr 2017;36:623-50. [Crossref] [PubMed]
- Ørum M, Gregersen M, Jensen K, et al. Frailty status but not age predicts complications in elderly cancer patients: a follow-up study. Acta Oncol 2018;57:1458-66. [Crossref] [PubMed]
- Ligthart-Melis GC, Weijs PJ, te Boveldt ND, et al. Dietician-delivered intensive nutritional support is associated with a decrease in severe postoperative complications after surgery in patients with esophageal cancer. Dis Esophagus 2013;26:587-93. [Crossref] [PubMed]
- Bower MR, Martin RC 2nd. Nutritional management during neoadjuvant therapy for esophageal cancer. J Surg Oncol 2009;100:82-7. [Crossref] [PubMed]
- Wie GA, Cho YA, Kim SY, et al. Prevalence and risk factors of malnutrition among cancer patients according to tumor location and stage in the National Cancer Center in Korea. Nutrition 2010;26:263-8. [Crossref] [PubMed]
- Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA 1999;281:1623-7. [Crossref] [PubMed]
- Daly JM, Weintraub FN, Shou J, et al. Enteral nutrition during multimodality therapy in upper gastrointestinal cancer patients. Ann Surg 1995;221:327-38. [Crossref] [PubMed]
- Scolapio JS. Decreasing aspiration risk with enteral feeding. Gastrointest Endosc Clin N Am 2007;17:711-6. [Crossref] [PubMed]
- Ott C, Ratiu N, Endlicher E, et al. Self-expanding Polyflex plastic stents in esophageal disease: various indications, complications, and outcomes. Surg Endosc 2007;21:889-96. [Crossref] [PubMed]
- Tahiri M, Ferraro P, Duranceau A, et al. Self-expanding metallic stent placement with an exaggerated 5-cm proximal tumor covering for palliation of esophageal cancer. Ann Gastroenterol 2015;28:347-52. [PubMed]
- Refai M, Andolfi M, Gentili P, et al. Enhanced recovery after thoracic surgery: patient information and care-plans. J Thorac Dis 2018;10:S512-6. [Crossref] [PubMed]
- Rubin GJ, Hardy R, Hotopf M. A systematic review and meta-analysis of the incidence and severity of postoperative fatigue. J Psychosom Res 2004;57:317-26. [Crossref] [PubMed]
- Whyte RI, Grant PD. Preoperative patient education in thoracic surgery. Thorac Surg Clin 2005;15:195-201. [Crossref] [PubMed]
- Lindström D, Sadr Azodi O, Wladis A, et al. Effects of a perioperative smoking cessation intervention on postoperative complications: a randomized trial. Ann Surg 2008;248:739-45. [Crossref] [PubMed]
- Tonnesen H, Rosenberg J, Nielsen HJ, et al. Effect of preoperative abstinence on poor postoperative outcome in alcohol misusers: randomised controlled trial. BMJ 1999;318:1311-6. [Crossref] [PubMed]
- Brady M, Kinn S, Stuart P. Preoperative fasting for adults to prevent perioperative complications. Cochrane Database Syst Rev 2003;CD004423. [Crossref] [PubMed]
- Ljungqvist O, Jonathan E. Rhoads lecture 2011: Insulin resistance and enhanced recovery after surgery. JPEN J Parenter Enteral Nutr 2012;36:389-98. [Crossref] [PubMed]
- Ljungqvist O, Nygren J, Thorell A. Modulation of post-operative insulin resistance by pre-operative carbohydrate loading. Proc Nutr Soc 2002;61:329-36. [Crossref] [PubMed]
- Yilmaz N, Cekmen N, Bilgin F, et al. Preoperative carbohydrate nutrition reduces postoperative nausea and vomiting compared to preoperative fasting. J Res Med Sci 2013;18:827-32. [PubMed]
- Smith MD, McCall J, Plank L, et al. Preoperative carbohydrate treatment for enhancing recovery after elective surgery. Cochrane Database Syst Rev 2014;CD009161. [Crossref] [PubMed]
- Yibulayin W, Abulizi S, Lv H, et al. Minimally invasive oesophagectomy versus open esophagectomy for resectable esophageal cancer: a meta-analysis. World J Surg Oncol 2016;14:304. [Crossref] [PubMed]
- Mariette C, Markar S, Dabakuyo-Yonli TS, et al. Health-related Quality of Life Following Hybrid Minimally Invasive Versus Open Esophagectomy for Patients With Esophageal Cancer, Analysis of a Multicenter, Open-label, Randomized Phase III Controlled Trial: The MIRO Trial. Ann Surg 2020;271:1023-9. [Crossref] [PubMed]
- Lohser J, Slinger P. Lung Injury After One-Lung Ventilation: A Review of the Pathophysiologic Mechanisms Affecting the Ventilated and the Collapsed Lung. Anesth Analg 2015;121:302-18. [Crossref] [PubMed]
- Gothard J. Lung injury after thoracic surgery and one-lung ventilation. Curr Opin Anaesthesiol 2006;19:5-10. [Crossref] [PubMed]
- Yang M, Ahn HJ, Kim K, et al. Does a protective ventilation strategy reduce the risk of pulmonary complications after lung cancer surgery?: a randomized controlled trial. Chest 2011;139:530-7. [Crossref] [PubMed]
- Jun IJ, Jo JY, Kim JI, et al. Impact of anesthetic agents on overall and recurrence-free survival in patients undergoing esophageal cancer surgery: A retrospective observational study. Sci Rep 2017;7:14020. [Crossref] [PubMed]
- Kim R. Anesthetic technique and cancer recurrence in oncologic surgery: unraveling the puzzle. Cancer Metastasis Rev 2017;36:159-77. [Crossref] [PubMed]
- Low DE, Allum W, De Manzoni G, et al. Guidelines for Perioperative Care in Esophagectomy: Enhanced Recovery After Surgery (ERAS®) Society Recommendations. World J Surg 2019;43:299-330. [Crossref] [PubMed]
- Umari M, Falini S, Segat M, et al. Anesthesia and fast-track in video-assisted thoracic surgery (VATS): from evidence to practice. J Thorac Dis 2018;10:S542-54. [Crossref] [PubMed]
- Marik PE, Cavallazzi R, Vasu T, et al. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med 2009;37:2642-7. [Crossref] [PubMed]
- Arieff AI. Fatal postoperative pulmonary edema: pathogenesis and literature review. Chest 1999;115:1371-7. [Crossref] [PubMed]
- Li C, Lin FQ, Fu SK, et al. Stroke volume variation for prediction of fluid responsiveness in patients undergoing gastrointestinal surgery. Int J Med Sci 2013;10:148-55. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Bass CS, et al. Fast tracking after Ivor Lewis esophagogastrectomy. Chest 2004;126:1187-94. [Crossref] [PubMed]
- Cheng DC. Pro: early extubation after cardiac surgery decreases intensive care unit stay and cost. J Cardiothorac Vasc Anesth 1995;9:460-4. [Crossref] [PubMed]
- Mandell MS, Lezotte D, Kam I, et al. Reduced use of intensive care after liver transplantation: influence of early extubation. Liver Transpl 2002;8:676-81. [Crossref] [PubMed]
- Salah M, Hosny H, Salah M, et al. Impact of immediate versus delayed tracheal extubation on length of ICU stay of cardiac surgical patients, a randomized trial. Heart Lung Vessel 2015;7:311-9. [PubMed]
- BED REST. thrombosis, and embolism. Lancet 1958;1:465-6. [PubMed]
- Convertino VA. Cardiovascular consequences of bed rest: effect on maximal oxygen uptake. Med Sci Sports Exerc 1997;29:191-6. [Crossref] [PubMed]
- Mayor MA, Khandhar SJ, Chandy J, et al. Implementing a thoracic enhanced recovery with ambulation after surgery program: key aspects and challenges. J Thorac Dis 2018;10:S3809-14. [Crossref] [PubMed]
- Zaouter C, Kaneva P, Carli F. Less urinary tract infection by earlier removal of bladder catheter in surgical patients receiving thoracic epidural analgesia. Reg Anesth Pain Med 2009;34:542-8. [Crossref] [PubMed]
- Hu Y, Craig SJ, Rowlingson JC, et al. Early removal of urinary catheter after surgery requiring thoracic epidural: a prospective trial. J Cardiothorac Vasc Anesth 2014;28:1302-6. [Crossref] [PubMed]
- Cheatham ML, Chapman WC, Key SP, et al. A meta-analysis of selective versus routine nasogastric decompression after elective laparotomy. Ann Surg 1995;221:469-76; discussion 476-8. [Crossref] [PubMed]
- Shackcloth MJ, McCarron E, Kendall J, et al. Randomized clinical trial to determine the effect of nasogastric drainage on tracheal acid aspiration following oesophagectomy. Br J Surg 2006;93:547-52. [Crossref] [PubMed]
- Mistry RC, Vijayabhaskar R, Karimundackal G, et al. Effect of short-term vs prolonged nasogastric decompression on major postesophagectomy complications: a parallel-group, randomized trial. Arch Surg 2012;147:747-51. [Crossref] [PubMed]
- Sato T, Fujita T, Okada N, et al. Postoperative pulmonary complications and thoracocentesis associated with early versus late chest tube removal after thoracic esophagectomy with three-field dissection: a propensity score matching analysis. Surg Today 2018;48:1020-30. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS. Results of a prospective algorithm to remove chest tubes after pulmonary resection with high output. J Thorac Cardiovasc Surg 2008;135:269-73. [Crossref] [PubMed]
- Johansson J, Lindberg CG, Johnsson F, et al. Active or passive chest drainage after oesophagectomy in 101 patients: a prospective randomized study. Br J Surg 1998;85:1143-6. [Crossref] [PubMed]
- Berkelmans GH, van Workum F, Weijs TJ, et al. The feeding route after esophagectomy: a review of literature. J Thorac Dis 2017;9:S785-91. [Crossref] [PubMed]
- Sun HB, Li Y, Liu XB, et al. Early Oral Feeding Following McKeown Minimally Invasive Esophagectomy: An Open-label, Randomized, Controlled, Noninferiority Trial. Ann Surg 2018;267:435-42. [Crossref] [PubMed]
- Ford SJ, Adams D, Dudnikov S, et al. The implementation and effectiveness of an enhanced recovery programme after oesophago-gastrectomy: a prospective cohort study. Int J Surg 2014;12:320-4. [Crossref] [PubMed]
- Pisarska M, Małczak P, Major P, et al. Enhanced recovery after surgery protocol in oesophageal cancer surgery: Systematic review and meta-analysis. PLoS One 2017;12:e0174382. [Crossref] [PubMed]
Cite this article as: Perroni G, Johnson C, Khandhar S, Veronesi G, Ambrogi V, Fernando HC. Implementation of eras for patients undergoing esophagectomy: a narrative review of the current literature and latest evidence. Curr Chall Thorac Surg 2021;3:37.


