
Electromagnetic navigational percutaneous transthoracic needle lung biopsy for peripheral small lung nodules not amenable to navigational bronchoscopy
Introduction
Lung cancer screening
Lung cancer is one of the most common causes of cancer deaths in the US, accounting for 1 in 4 cancer related deaths. With detection of early stage lung cancer, the 5-year survival rate can be >90% (1). On March 9th 2021, The American Cancer Society updated their lung cancer screening guidelines. The first major change was that the age to start screening was decreased from 55 to 50 years of age. The second is that the pack years was decreased from 30 to 20. Due to these two changes, there will be an increasing number of patients screened. Increased screening will therefore increase the incidence of peripheral lung nodules, which are defined as lesions <3 cm in size, found on low dose computed tomography (CT) scan, and consequently increase the number of peripheral lung nodule evaluations (2). The patients who are found to have lung nodules detected on screening will then be triaged by risk of malignancy with tools such as stratifications in the Fleishner society statement (3). There is a 20% reduction in the lung cancer mortality in high risk patients who undergo low dose CT scans for cancer screening based on the National Lung Cancer Screening Trial (NLST) (1). Early diagnosis of lung cancer is imperative as the cure rate for Stage I lung cancer can be as high as 88%. The International Early Lung Cancer Action Program (I-ELCAP) study showed better cancer-specific survival in stage I non-small cell lung cancer (NSCLC) that was resected within 1-month of diagnosis (4). This data urges us to get prompt diagnosis of SPNs.
Lung cancer diagnosis
There are two major minimally invasive modalities for tissue diagnosis: bronchoscopic intervention or transthoracic needle biopsy (TTNB) guided by CT, ultrasound, or fluoroscopy. In general, CT-guided TTNB is preferred for nodules located close to the chest wall and far away from vessels. These are usually biopsied by interventional radiologists (IR). Bronchoscopic intervention is favored for nodules located in proximity to a patent bronchus as well as in individuals who are at high risk for pneumothorax following TTNB.
As mentioned above, bronchoscopic guided biopsies can be done for more central lesions. There is a decreased chance of pneumothorax in bronchoscopic guided biopsies but in some reports, an increased chance of hemorrhage, especially in patients who are on anti-platelet medications. Although the bronchoscopic yield for solitary pulmonary nodules (SPN) historically remains low (Table 1), some advantages to bronchoscopy include a lower complication rate (14) as well as the ability to stage the disease at the time of diagnosis with mediastinal and hilar lymph node sampling with endobronchial ultrasound (EBUS) (13).
Table 1
| Complication/procedure | Pneumothorax | Pneumothorax requiring CT insertion | Hemorrhage | Diagnostic yield (SPN <2 cm) | Diagnostic yield (SPN 22±10 mm) |
|---|---|---|---|---|---|
| CT-guided biopsy | 15–42% (5-8) | 4–17.4% (5,8) | 10% (9) | 85–92% (9) | |
| Flexible bronchoscopy + TBNB | 4.9% (10) | 47–66% (9,11,12) | |||
| Navigational bronchoscopy + TTNB | 0–21% (6,10-13) | 8–12.4% (6,11-13) | 1.6% (13) | 81–90% (6,10,13) |
CT, computed tomography; SPN, solitary pulmonary nodule; TBNB, transbronchial biopsy; TTNB, transthoracic needle biopsy.
CT-guided lung biopsies do require radiation when compared to transbronchial biopsy (TBNB), although this is similar to that of navigational techniques. In the literature, there is a higher rate of pneumothorax, especially in patients with emphysematous lungs, with a small percentage of these requiring intervention. For example, in one study, there was a 25.3% rate of pneumothorax with 5.6% requiring intervention (5). The increased pneumothorax rate is likely from the need for multiple pleural punctures in order to make a real time diagnosis and increase the diagnostic yield (13). Hemorrhage is another reported complication. Patient and nodule characteristics most often mentioned as risk factors are older age, presence of emphysema, smaller lesion size, increased lesion depth, non-pleural contact, and smaller pleural-needle angle (5).
For electromagnetic navigational (EMN) bronchoscopy (ENB), this may require same day CT imaging which increases radiation exposure to the patient, which is similar to that of a CT-guided IR biopsy, but is negligible in the grand scheme of diagnosing a possible lung cancer. One advantage of same day CT imaging is the ability to identify changes in the lesion. If the lesion is smaller or no longer present, the procedure maybe be cancelled which would reduce the need for an unnecessary procedure and in turn reduce the risks associated with the procedure (15).
The sensitivity of TTNB is greater than that of bronchoscopy with TBNB. CT-guided biopsy is still the gold standard for diagnosis of peripheral SPNs. Guidelines for establishing the diagnosis of lung cancer by the ACCP state “In patients with peripheral lung lesions difficult to reach with conventional bronchoscopy, electromagnetic navigation guidance is recommended if the equipment and the expertise are available (grade 1C). Remark: the procedure can be performed with or without fluoroscopic guidance and it has been found complementary to radial probe ultrasound” (16). Unfortunately, navigational bronchoscopy [EMN and virtual bronchoscopic navigation (VBN)] combined with “real time tools in target confirmation technology” such as 2D fluoroscopy, CT fluoroscopy, cone beam CT, radial probe-EBUS has provided limited success to salvage diagnostic yield rates. With the advent of ENB guided biopsy, the diagnostic yield for bronchial biopsies has increased. In a cohort study by Bhatt et al. (9), CT-guided sampling is more likely to be diagnostic then ENB guided biopsy (85% vs. 66%). But when ENB is combined with percutaneous needle biopsy, the diagnostic yield increases.
In order to improve diagnostic yield rates, several methods can be combined. Transbronchial biopsies, navigational biopsy, EBUS, and TTNB and aspiration can be done in the same procedure. A feasibility study was done by Yarmus et al. (6) where they combined navigational biopsy with transthoracic biopsy and noted that this was feasible in 96% of their cases with a 21% pneumothorax rate and 8% requiring chest tube placement. When this was combined with EBUS, the diagnostic yield was 92%. This study showed that it is acceptable, safe, and feasible to combine these procedures (6,11,12). Another study showed similar results with diagnostic yields for patients undergoing percutaneous biopsy being 90% and when combined with EMN was 92%; an increase in diagnostic yield by 20% when adding percutaneous needle biopsy has also been shown (17,18).
In a different study, retrospective analysis was done to look at diagnostic yields of tip tracking instruments for the localization of the peripheral lung nodules using navigational bronchoscopy. The average size nodule was 22±10 mm. This system provided effective yields of diagnosis of these peripheral lung nodules with a yield of 90% with no pneumothoraces or complications reported (10).
Navigational bronchoscopy has improved access to peripheral pulmonary nodules, ones that were not previously amenable to TBNB. Now with the advent of tip tracked technology diagnostic yields have improved. This system also allows fiducial marker placement (19).
Recent advancements in technology enable non-IR providers to apply an ENM system for percutaneous TTNB for peripheral lung nodules. This can be done in a single procedural setting with EBUS, ENB, and TTNB and can be staged in a procedural fashion using rapid onsite cytology evaluation (ROSE).
ENB
Two ENB systems are commercially available in US. The SuperDimension (Medtronic, Minneapolis, MN, USA) released in 2004 and the SPiNDrive (Veran Medical Technologies, Inc, St Louis, MO, USA) released in 2010 with subsequent release of the VERAN SpinPerc system in 2013 to the US market. The first clinical SpinPerc case was done in 2014 and the first human prospective 24 case pilot study using the VERAN SpinPerc platform was done by Yarmus et al. and reported in 2016 (6). They demonstrated an 83% diagnostic yield rate with 21% pneumothorax rate. Mallow et al. did a retrospective multicenter analysis of 129 cases of pulmonologist performing percutaneous transthoracic lung biopsies using the SpinPerc platform, they demonstrated an 81.1% diagnosed yield rate with 17.8% pneumothorax rate (13).
Both systems use a dedicated specific CT protocol to maximize segmentation of the airway. The superDimension system requires an inspiratory scan without any special sensors while the VERAN SPiNDrive program requires an inspiratory and expiratory scan with special sensor pad (v Pads); this sensor provides automatic registration and is able to track moving nodules with “respiratory gating” during navigation.
The superDimension system requires a “location board” to be placed under the patient for the electromagnetic field generator as well as a designated operating room (OR)/procedural room in which pre-field bed and room mapping. The VERAN SPiNDrive system requires the v Pads and a mobile field generator tower that can be placed in any room of the hospital.
The superDimension system is compatible with a therapeutic scope using a steerable extended working channel catheter system. The VERAN system is compatible with a therapeutic scope (6.3 mm) and a peripheral scope (4.2 mm) with a channel larger than 2.0 mm. The superDimension system has a navigation sensor in order to localize the lesion. Once the lesion is localized, the sensor is removed and the biopsy tools are advanced. The VERAN SPiNDrive instruments have built-in electromagnetic sensors at the tip allowing the lesions to be localized during sampling.
VERAN SPIN PerC system allows for transition from navigational bronchoscopy to navigated trans-thoracic needle biopsy or aspiration in the same procedure setting. This does not require any extra radiation as the percutaneous biopsy is done with the EMN system. This would be pursued if navigational bronchoscopy did not yield a definitive diagnosis by ROSE or frozen section evaluation.
Transthoracic navigational bronchoscopy procedure
A chest CT scan with placement of a navigational tracking sensor pad (v Pads) is placed on the patient’s chest wall (anterior or posterior). The v Pad placement, position of the patient in the CT scanner and positioning during the procedure will depend on the location of the target lesion. If the approach for the percutaneous needle biopsy is anterior, then the patient is placed supine in the CT scanner with pad placement on anterior chest and a supine navigational bronchoscopy procedure and vise versa for a posterior approach (Figure 1). A lateral approach can be used for patients who require video-assisted thoracoscopic surgery (VATS) nodule localization, this procedure will not be discussed in this review.

After the navigational bronchoscopy procedure, if no diagnosis has been made, the navigation system can be switched to the percutaneous transthoracic biopsy mode. The site for skin puncture is guided by the navigation system. The electromagnetic tip tracked biopsy needle introducer (19-gauge × 105 mm, 17-gauge × 155 mm) is then used and is advanced into the target lesion under navigational guidance. The biopsy core needle is rotated after each biopsy to obtain samples from different areas of the lesion while keeping the introducer in skin and removing and re-inserting the needle, reducing the need for multiple puncture sites. In general, the incidence of the post procedural pneumothorax is the same as conventional CT-guided percutaneous transthoracic needle lung biopsy. The risk factors for pneumothorax are emphysema, depth of lesion from the pleura, and lesion size. Independent risk factors for pneumothorax were no prior pulmonary surgery, lesions in the lower lobe, greater lesion depth, and a needle trajectory angle of <45° (20). On the contrary, gender, age, and the number of pleural passes has not been shown to correlate with the incidence of pneumothorax (21). If there is a suspicion for a developing pneumothorax based on the difficulty of the procedure, physical exam or the ventilator settings, an ultrasound (US) can be used to identify a pneumothorax in the procedure room, and if noted, a chest tube can be placed in the procedure room. If the US does not show a clear pneumothorax, the presence or absence of a clinically relevant pneumothorax can be confirmed with a portable chest X-ray (CXR) which can be done at bedside in the OR/procedure room.
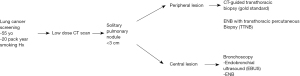
Algorithm for the evaluation of SPNs
With the new screening guidelines for lung cancer with low dose CT scans being done for patients over 55 years of age with a 20-pack year smoking history there will be more SPNs (nodules <3 cm) found incidentally. For proximal central lesions, EBUS can be used to biopsy these. For central lesions that are more distal, ENB technology is used to obtain biopsies. For peripheral lesions, the gold standard in CT-guided biopsy. Often in patients who are high risk for hemorrhage or pneumothorax CT-guided biopsies cannot be performed. With the advent of ENB with the ability to do transthoracic percutaneous biopsies, as these procedures are done in the operating room with the patients under general anesthesia and intubated, TTNB can be attempted on these higher risk patients (Figure 2).
Conclusions
The advent of the electro-magnetic navigation guided transthoracic percutaneous biopsy system increased the diagnostic yield during TBNB, EBUS and navigation bronchoscopy procedures when the diagnosis is not made. This gives more options for diagnosis then CT-guided IR biopsy and has a similar diagnostic yield with a similar or improved complication rate when compared to CT-guided biopsy, which is the current gold standard.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Scott Swanson, Daniel Dolan) for the series “How to Evaluate, Diagnose and Treat Small Lung Nodules” published in Current Challenges in Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts-20-106/coif). The series “How to Evaluate, Diagnose and Treat Small Lung Nodules” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sakurai H, Nakagawa K, Watanabe S, et al. Clinicopathologic features of resected subcentimeter lung cancer. Ann Thorac Surg 2015;99:1731-8. [Crossref] [PubMed]
- US Preventive Services Task Force. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021;325:962-70. [Crossref] [PubMed]
- Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology 2013;266:304-17. [Crossref] [PubMed]
- Flores R, Bauer T, Aye R, et al. Balancing curability and unnecessary surgery in the context of computed tomography screening for lung cancer. J Thorac Cardiovasc Surg 2014;147:1619-26. [Crossref] [PubMed]
- Heerink WJ, de Bock GH, de Jonge GJ, et al. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol 2017;27:138-48. [Crossref] [PubMed]
- Yarmus LB, Arias S, Feller-Kopman D, et al. Electromagnetic navigation transthoracic needle aspiration for the diagnosis of pulmonary nodules: a safety and feasibility pilot study. J Thorac Dis 2016;8:186-94. [PubMed]
- Wiener RS, Schwartz LM, Woloshin S, et al. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med 2011;155:137-44. [Crossref] [PubMed]
- Cox JE, Chiles C, McManus CM, et al. Transthoracic needle aspiration biopsy: variables that affect risk of pneumothorax. Radiology 1999;212:165-8. [Crossref] [PubMed]
- Bhatt KM, Tandon YK, Graham R, et al. Electromagnetic Navigational Bronchoscopy versus CT-guided Percutaneous Sampling of Peripheral Indeterminate Pulmonary Nodules: A Cohort Study. Radiology 2018;286:1052-61. [Crossref] [PubMed]
- Flenaugh EL, Mohammed KH. Initial Experience Using 4D Electromagnetic Navigation Bronchoscopy System With Tip Tracked Instruments For Localization of Peripheral Lung Nodules. The Internet Journal of Pulmonary Medicine 2016;18:
- Arias S, Lee H, Semaan R, et al. Use of Electromagnetic Navigational Transthoracic Needle Aspiration (E-TTNA) for Sampling of Lung Nodules. J Vis Exp 2015;e52723. [Crossref] [PubMed]
- Wang N, Ma H, Huang H, et al. Electromagnetic Navigation Bronchoscopy Combined Endobronchial Ultrasound in the Diagnosis of Lung Nodules. Medicine (Baltimore) 2021;100:e23979. [Crossref] [PubMed]
- Mallow C, Lee H, Oberg C, et al. Safety and diagnostic performance of pulmonologists performing electromagnetic guided percutaneous lung biopsy (SPiNperc). Respirology 2019;24:453-8. [Crossref] [PubMed]
- MacDuff A, Arnold A, Harvey J, et al. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65:ii18-31. [Crossref] [PubMed]
- Semaan RW, Lee HJ, Feller-Kopman D, et al. Same-Day Computed Tomographic Chest Imaging for Pulmonary Nodule Targeting with Electromagnetic Navigation Bronchoscopy May Decrease Unnecessary Procedures. Ann Am Thorac Soc 2016;13:2223-8. [Crossref] [PubMed]
- Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e142S-65S.
- Moore C, Whang B, Wiener D, et al. Electromagnetic navigational (EMN) bronchoscopy and EMN percutaneous transthoracic needle biopsy of the chest lesion: the first 102 consecutive early experience cases. Chest 2019;156:A1678. [Crossref]
- Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012;142:385-93. [Crossref] [PubMed]
- Belanger AR, Burks AC, Chambers DM, et al. Peripheral Lung Nodule Diagnosis and Fiducial Marker Placement Using a Novel Tip-Tracked Electromagnetic Navigation Bronchoscopy System. J Bronchology Interv Pulmonol 2019;26:41-8. [Crossref] [PubMed]
- Hiraki T, Mimura H, Gobara H, et al. Incidence of and risk factors for pneumothorax and chest tube placement after CT fluoroscopy-guided percutaneous lung biopsy: retrospective analysis of the procedures conducted over a 9-year period. AJR Am J Roentgenol 2010;194:809-14. [Crossref] [PubMed]
- Zhou Q, Dong J, He J, et al. The Society for Translational Medicine: indications and methods of percutaneous transthoracic needle biopsy for diagnosis of lung cancer. J Thorac Dis 2018;10:5538-44. [Crossref] [PubMed]
Cite this article as: Lighter M, Tsukada H. Electromagnetic navigational percutaneous transthoracic needle lung biopsy for peripheral small lung nodules not amenable to navigational bronchoscopy. Curr Chall Thorac Surg 2022;4:35.


