Boosting lung transplantation, a narrative review of the impact of donor after cardiac death, organ care system and ex vivo lung perfusion in expanding donor pool
Introduction
Lung transplantation (LTx) is the proven definitive treatment for some of the most serious chronic debilitating diseases of the lungs, such as, cystic fibrosis, chronic obstructive pulmonary disease, pulmonary hypertension, and idiopathic pulmonary fibrosis. Donor lungs are scarce, and as a result, people continue to die or deteriorate on the waiting list before undergoing LTx (1). Thus, there is a challenge to optimize the selection and organ allocation based on clinical severity and existing pathology. Trying to solve this problem, scientists and physicians are shifting the donor criteria to a more “aggressive” attitude regarding the available lung organs in the donor pool by expanding the selection criteria to involve more marginal lungs. In order to expand the donor pool safely, scientists have created new tools to assist in the proper observation, evaluation, and potential reconditioning of these “marginal” lungs (2-4). We will discuss these innovative tools and techniques below. We present the following article in accordance with the Narrative Review reporting checklist (available at https://ccts.amegroups.com/article/view/10.21037/ccts-21-25/rc).
Methods
We reviewed impactful trials and studies that have been published in English literature over the last two decades and how their results altered the management of LTx patients. PubMed, OVID and Google Scholar were queried for all articles involving the terms: lung transplant, donor criteria, Donation after Circulatory Death (DCD), and ex vivo lung perfusion, from 01/02/2000 to 02/16/2022. Any article with historical significance or relevance to emerging LTx technology were included. Only articles published in English or translated into English language were included, excluding other languages articles (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | 02/16/2022 |
| Databases and other sources searched | PubMed, OVID and Google Scholar |
| Search terms used | Lung transplant, donor criteria, Donation after Circulatory Death (DCD), and ex vivo lung perfusion |
| Timeframe | 01/02/2000 to 02/16/2022 |
| Inclusion criteria | English literature |
| Selection process | Independently |
Historically
LTx has developed in conjunction with vascular anastomotic techniques, cardiopulmonary bypass support, and an understanding of immunologic barriers. The earliest reports of experimental LTx are those of Demikhov, who in 1947 performed individual canine lung lobe transplantation (5,6). This was followed by Dr. James Hardy, at the University of Mississippi. He performed the first human lung transplant and is credited with early investigation of immunosuppressive therapy to increase lung allograft survival rate in dogs (7). On June 11th of 1964, the first human lung transplant procedure was performed on 58-year-old John Russel after being admitted to University of Mississippi Medical center with severe emphysema and an obstructing carcinoma of the left mainstem bronchus. Disappointingly, Mr. Russel lived only 18 days and died of multi-organ failure (7 Hardy). This was followed by several other attempts over a 20-year period until the first successful single lung transplant was reported by Joel Cooper at the University of Toronto (8). The success of that procedure was attributed to both the introduction of cyclosporin and the use of omental pedicle grafts to supply blood to the tracheal anastomosis.
Following rapid advancement in the surgical techniques, postoperative care, and immunological management of solid organ transplantation, large numbers of referrals of patients to the waiting list overwhelmed the limited supply of the donor organs. This was further exacerbated by the fact that only around 20% of donors have a suitable lung for donation according to the standard lung donor criteria (1). We are now faced with a long waiting list of recipients, and that leads to an increase in wait-list mortality, clinical deterioration of a patient’s condition beyond transplantation benefit, or increasing the risk of morbidity and mortality of a delayed surgery.
Out of necessity, scientist and physicians are trying to solve this problem by increasing the total number of donor lungs available and by improving medical treatment of potential recipient’s lung diseases so they have a slower progression to end-stage disease. Scientist and physicians have discovered creative and bold avenues to pursue and obtain the aforementioned desired results by increasing the total number of successful LTxs, decreasing the waitlist mortality, and providing a better chance for those on the waiting list to obtain their lung transplant before comorbidities accumulate (2-5,9,10). In this review, we will explain that increasing the donor pool has been achieved by accepting organs from controlled circulatory death donors in conjunction with the use of new donor organ assessment techniques, like Ex Vivo system (XVIVO Perfusion AB, Goteborg, Sweden) and organ care systems (OCS).
Donation after brain death (DBD)
DBD also known as heart-beating donors, are derived from patients who have suffered brain stem death (10,11). These patents are usually intensive care unit (ICU) based patents in an apneic coma of known etiology. The most likely causes of brainstem death is trauma or intra-cerebral hemorrhage. In these donors, the heart remains beating and the body remains perfused with oxygenated blood. Thus, the risk for a warm ischemic injury to the organs is minimal prior to the procurement.
DCD
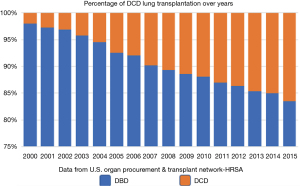
There has been a steady increase of the absolute number LTxs in the past decade performed in the United States this was associated with steady increase in LTx from DCD donors (Figure 1). Furthermore, there has been a slight decrease in waiting list mortality to 15% per year (12). This indicates that lung transplants following DCD have increased over the past 5 years from 1% to 4% in 2017 (13,14).
The first LTx was performed with a DCD donor (15), with the practice regaining interest during 1995 with the publication of a case study by D’Alessandro and coworkers. The publication reported the first successful DCD donor lung transplant as part of an institutional DCD program (16), using controlled DCD [i.e., DCD donors after withdrawal of life support (WLS) in ICU, Maastricht DCD category 3] (17,18).
The lung is a unique organ with a relatively low metabolic requirement and has 2–3 hours of tolerance of warm ischemia (19). Such tolerance allows for prospect of DCD donors in several scenarios, including “controlled” and “uncontrolled” situations, characterized by the 1995 Maastricht categories. In a controlled situation (Category III; withdrawal of treatment, Category IV; unexpected cardiac arrest during brain-death donor procurement), a decision to withdraw life-support treatment is made and death is predicted, with organ donation consent readily obtained from the family. While in uncontrolled situation (Category I; dead on arrival, Category II; failed resuscitation), death is sudden and unexpected, and it usually happens outside the hospital or in an emergency room (20).
In order to understand the concept of DCD we should understand the ethical and clinical guidelines which allow us to procure organs from donors. The Uniform Determination of Death Act (UDDA); Stated that “an individual who has sustained either irreversible cessation of circulatory and respiratory functions or irreversible cessation of all functions of the entire brain, including the brain stem, is dead.” (21). A determination of death must be made in accordance with accepted medical standards. Legally, an individual who is brain dead but has intact circulation and ventilation can be considered legally dead.
DCD offer a new source of viable lungs for transplantation. However, even in a Maastricht category III donor (controlled DCD), the common category of DCD lung procurement, there are limitations. Such limitations include the short time available after the declaration of death to begin procurement and preservation of the organ. Furthermore, withdrawal of life-support and death certification may typically be performed in the ICU while the lungs are procured in an operating room (OR). In such donor cases, the heart stops for a period of time before the lungs are harvested and cooled, which leaves a critical time for ischemic injury to start. Such controlled cases are called non-heart-beating donors, but it is not the only category for DCDs.
Controlled DCD
Controlled DCD cases typically involve ICU patients who suffered from a global neurological injury of the cerebral hemispheres while retaining basic function of the brain stem allowing them to maintain proper ventilation and basic limited reflexes. A medical decision is reached about the unlikely of recovery by the medical staff and, with the legal approval of the family of the patient, the withdrawal of treatment can be planned (17,21). After cessation of the heartbeat, the patient is rapidly transferred to the operating theatre where an urgent sternotomy is performed, and the lungs are flushed and cooled in situ. This can be done with a relatively short period of warm ischemia less than 15 minutes.
Uncontrolled DCD (uDCD)
uDCD cases typically involve patients who have died suddenly and unexpectedly of a cardiac event or from trauma (21). These donors are often found in the Emergency Department. Following repeated failures at resuscitation, the patient is declared dead, and a brief ‘hands-off’ period begins to ensure no spontaneous return of the circulation. Afterward, the vascular system is cannulated peripherally, and the organs are perfused in-situ by connecting the cannulas to a cardiopulmonary bypass machine in what we called normothermic regional perfusion (NRP). In uDCDs, the duration of warm ischemia is greater and may include additional partial ischemia during attempts at cardiopulmonary resuscitation. Nonetheless, it is estimated that the human lung can sustain a period of 2–3 hours of absolute warm ischemia and still recover function provided ventilation and oxygenation is maintained during the ischemic period (22). uDCD is an expanding area of practice in multiple transplant centers around the world, aiming to increase the donor pool beyond controlled DCD (13,23). Madrid group reported consistent encouraging results with uDCD LTx (24). The group used an acceptable procedural criterion using cold infusion of topical Perfadex solution and pulmonary artery/left atrium blood gas measurement after acceptable visual inspection to donor lungs. They reported a similar one-year survival compared to results in their DBD program, but they also reported a higher incidence of graft dysfunction in the uDCD recipients.
Furthermore, the Toronto Group recently published their experience in Lung Transplant from uDCD lung donation, and they described the successful use uDCD donors using a simple method of in situ lung inflation to protect the organ from warm ischemic injury (25). The Toronto group, in contrast to other European methods, used in situ donor lung preservation for uDCD where no reinstitution of circulation (via NRP, or continuous chest compressions) is performed and only simple measures of lung protected ventilation are initiated after declaration of death. This simple method expands the pool for the use of uDCD alleviating the possible ethical concerns raised by the initiation of blood recirculation (NRP) in potential organ donors. The process of evaluating the donor, securing the Organ Donation Organization and the family approval for allocating the organ typical requires 90 min. Following the approval, the donor enters a clinical protocol where the lungs are re-intubated and inflated with CPAP while waiting for the retrieval process to begin (which should be not more than 3 hours from death). After that, the lung is transported to the transplant center and put on ex vivo lung perfusion (EVLP) machine for evaluation and management before decision is made to proceed with the transplant surgery.
Donor criteria for DCD donation
DCD donor criteria is the same as DBD donor criteria (i.e., standard criteria), although some centers now include donors from the category of an “extended criteria” after the introduction of the technique of EVLP which help to monitor, assess, and in some cases intervene to improve the condition of the donated lung to make suitable for transplantation.
Important issues to be discussed before controlled DCD lung donation
Heparin administration and timing
Donor hospitals differ in their guidelines regarding when and where heparin should be administered to the DCD donor. Ideally, heparin must be administered in a beating heart donor i.e., before the WLS to achieve the best results physiologically for metabolism and distribution. This should be discussed in advance with the hospital staff and the procurement team should be prepared to encounter different hospital policies. Some centers in the United States refuse to proceed in the procurement process if a donor hospital refuses to administer heparin.
Timing and location of WLS process?
This is an important issue to discuss before proceeding in the procurement process starts since warm ischemic time (WIT) should be minimized at all costs. WLS includes cessation of all pharmacological and mechanical life support including extubation of the endotracheal tube, and this process is better done in the OR or a nearby ICU to minimize the period between donor death certification and starting the procurement process.
Agonal and warm ischemia waiting time
Agonal time is the duration between WLS and expiration of the donor. This might last minutes or hours. During this period the donor circulation will be kept intact. There may be periods of time, however, where the minimum circulation needed to properly perfuse an organ is not achieved, and this may affect viability of the lung. A rapid decline in physiologic status is more desirable than slow progression. There is currently no concrete data to determine the safest duration for agonal time regarding the lungs. Some centers set a time limit to wait until expiration. Because the lung has a unique physiological criterion that can help it withstand longer ischemic times (such as dual blood supply and additional source of oxygen supply directly from the alveolar gas), waiting time might be extended to 2 hours before a recovery team declines the organ, provided the donated organ is kept within the minimum physiological status in ventilation and perfusion. This can be sustained as long as the donor’s blood pressure is above 70–80 mmHg and/or saturation above 80% and the lungs are protected from aspiration by nasogastric aspiration of stomach content before WLS started. If those criteria are not met during the agonal period for more than 5 minutes and still the hospital staff cannot certify death according to their standards, the procurement team should decline the organ and explain the rationale behind their decision.
Endo-tracheal tube (ET) removal (extubation)
Most of the donor hospitals and organ procurement organization (OPO) centers state that the ET must be removed at the start of the withdrawal process because the ET prevents the “natural” process of donor expiration by preventing the collapse of the trachea and keep the airways open. The presence of the ET after withdrawal is technically more beneficial to the procurement process than removing it because of two reasons, first; it will protect the lung from aspiration during the agonal phase, and secondly; it will nullify the extra time needed to re-intubate the donor and restore full inflation of the lung. In certain situations, the reintubation process is a burden if it is conducted by a less experienced staff from the donor hospital once the donor arrived at the OR room. Since the usual scenario is the removal of the ET, the procurement team must be prepared to re-intubate the donor immediately after death pronounced either by a well-trained staff from the donor hospital or by the procurement team themselves. To minimize accidental aspiration, the procurement team must insist on keeping the stomach empty by keeping the nasogastric tube in with continuous suctioning during the expiration process until reintubation performed.
Initial WIT
This is the period between announcing the death of the donor and starting the antegrade flush, which is usually should be kept less than 30 min. Nevertheless, longer periods have been reported with good results, but not more than 60–90 min (26).
Techniques of DCD lung procurements
Here we describe the most commonly used techniques in the USA, variations were described when needed.
- The donor received heparin (30,000 IU) before extubation and withdrawal of all pressor support.
- Nasogastric tube is placed before extubation to evacuate the stomach and is then removed after reintubation.
- Waiting for one to two hours (depending on donor hospital protocol and local organizing OPO guidelines) for cardiac arrest and cessation of electrical activity. If cardiac arrest does not occur in that time frame, the patient is reintubated and returned to the ICU. If cardiac arrest does occur, a member of the donor hospital certifies death, waiting for 5 minutes after that to be sure there is no spontaneous recovery. Then, a skin incision made, and the specific steps of organ procurement are initiated.
- The donor is reintubated and ventilated at 7 mL/kg at FiO2 of 100%. If not yet performed, bronchoscopy is carried out to evaluate the donor organ, examine the condition of the airways and suctioning of secretions if present, by the procurement team or the donor hospital staff.
- Simultaneously, the donor is prepared and draped in a standard fashion followed by median sternotomy.
- A pulmonary artery perfusion cannula is placed, the left atrial appendage is amputated, and 4 L of cold antegrade low potassium dextran solution (Perfadex; Vitrolife AB, Kungsbacka, Sweden) with prostaglandin E1 (500 Mcg) is injected directly into the pulmonary artery if it wasn’t already added to the first flush bag (27).
- The superior vena cava (SVC) is clamped and the inferior vena cava (IVC) is partially transected to drain the warm blood from the right side of the heart and prevent it from returning to the pulmonary circulation.
- The aorta is cross clamped even if the heart is not procured. This will stop the right ventricle from pumping warm blood to pulmonary circulation and prevent the retrograde influx of the intraabdominal flushing solutions.
- The pleural spaces are widely opened to expose the lungs, and gross visual inspection of the lungs is performed, followed by topical cooling with slushed ice solution.
- After finishing the antegrade flush and the donor lungs then fully mobilized, the trachea is stapled after a gentle respiratory recruitment maneuver, the lungs are then removed en-block
- Back table retrograde flushing is then carried out using 250 mL of Perfadex solution in each pulmonary vein orifice, followed by final inspection of the lungs and packaging on ice before transportation.
Current practices involving DCD
The shortage of donor lungs suitable for transplantation forced transplant teams to seek alternate avenues to obtain lungs for transplant. As mentioned above, only 20–25% of the donated lungs are suitable for transplantation, and this compares poorly to other solid organs. DCD has helped overcome this shortage by 5–10% in some centers, and that number was calculated before introducing the concept of ex vivo donor lung management (28). Now that we can test for transplant suitability after the lungs have been harvested, it will encourage more centers to consider this resource. This should lower waiting times on the lung transplant list as there will be more lungs available for transplant. The new systems will give more confidence to transplant physicians and surgeons, which will allow them to utilize this new source of organs suitable for transplant. Both the OCS and Ex Vivo platforms are appropriate. Most centers have established their own protocols and guidelines to procure these organs with the help of one of these EVLP techniques. These techniques have decreased waiting list times, morbidity, and mortality at several centers (29,30).
An article by Van Raemdonck et al. (13) examined the International Society for Heart and Lung Transplantation (ISHLT) Thoracic Transplant Registry data for Lung Transplantation patients between 2003 and June of 2017 at 23 transplant centers participating in the DCD registry across Australia, Europe, and North America. The study included 11,516 LTx, with 9.5% of these transplants designated as DCD transplants. Furthermore, the study’s results also observed an upward trend in DCD transplants from 0.6% in 2003 to 15.2% in 2017. The 5-year survival rates amongst DCD and DBD were also found to be comparable. These findings support measures to increase DCD derived LTxs (30).
Lung donors’ management utilizing OCS
Originally, donor lungs were transported using cold storage in preservation solution at low temperature (around 4c) and they had to be delivered within a limited time frame (~7 hours). This method necessitates high quality lungs (standard criteria) and short transport times in order to minimize ischemic damage. Furthermore, ischemia-reperfusion injury damages the donor lung and can produce primary graft dysfunction (PGD) in severe instances (31). A severe consequence of PGD, Chronic Lung Allograft Dysfunction subjects the patient to poor outcomes and quality of life (32). Cold-stored lung transport technique limits clinician’s ability to assess the suitability of lung for transplant after procurements. While a given lung may appear high quality during procurement, the quality of the lung may have declined during transport, and this could lead to PGD after transplantation into the recipient.
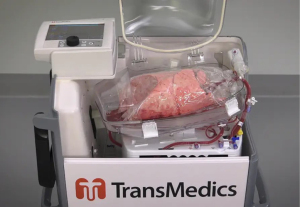
The portable OCS (TransMedics, Inc., Andover, MA, USA) (Figure 2) is described as an advanced portable EVLP system. The OCS allows the explanted lungs to be preserved with warm physiologic perfusion and ventilation atmosphere during transport. This normothermic perfusion minimizes cold ischemic time and improves the length of time which an organ is still viable for transplant.
The effectiveness of the OCS has been assessed in multiple studies, namely INSPIRE and EXPAND (33,34). The INSPIRE trial was the first prospective, randomized controlled study for standard-criteria bilateral LTx. INSPIRE specifically compared transplantation outcomes in patients who received donor lungs preserved through the OCS system to those who received lungs through the traditional cold storage method. The primary endpoint was identified as the absence of PGD grade 3 within the first 72 hours post-op along with 30-day survival. This endpoint was met in 79.4% of OCS patients and 70.3% of control patients. Additionally, OCS lung recipients showed shorter ventilation time, ICU time, and hospital stay time. These last results were not statistically significant but were clinically meaningful (33).
The EXPAND trial was a prospective single-arm study, aimed to show the safety and efficacy of portable EVLP preservation for 79 transplanted lungs from Extended Criteria Donors (ECD) or DCD donors. The study showed the incidence of PGD 2 or 3 being 16% at 72 h post transplantation and the 30-day mortality being 1%, indicating favorable results for the population groups. The primary endpoint was identical to the INSPIRE study, this endpoint was achieved in 54% of patients, with the majority of patients who failed to meet endpoint exhibiting PGD grade 3. Portable OCS preservation resulted in 87% lung donor use in transplantation, with many lungs having previously been rejected for usage in other transplant centers. Despite not having a direct comparison to preservation by cold storage, the study showed promise in the ability of OCS to expand the availability and accessibility of LTx (35).
Donor lung preservation while on OCS
During OCS lung perfusion, vascular resistance and airway pressures are monitored to assess the stability of perfusion conditions. OCS Lung System allows for lung assessment during preservation by using blood gas analyzer to measure the lung’s blood pO2 level, pulmonary artery pressure measurement, pulmonary vascular bed resistance, and lung compliance. To rest the OCS donor lung, it is perfused with oxygenated blood perfusate, and protective ventilation settings are utilized. Cold ischemic times are limited to a short period during donor procurement, lung instrumentation on OCS, and surgical re-implantation (36).
OCS and PGD
Transporting donor lungs in OCS could attenuate the effects of ischemia-reperfusion injury that resulted from long period of cold ischemia. The results of the INSPIRE trial, showed that the OCS Arm was associated with a reduction in the incidence of PGD grade 3 (PGD3) within the initial 72 hours. Furthermore, the study showed that there is no difference in PGD3 at the latest time-point (T72) between the OCS and Control groups (33).
OCS in DCD
Problems may arise during procurement of DCD organs, including hypoxia and hypotension, which might limit future widespread utilization. Limitations also include reduced, or even absent, organ perfusion for a period of time leading to ischemia (and re-perfusion injury syndrome later) during progression to circulatory arrest (agonal phase). Hypoperfusion induces proinflammatory sensitization response which may lead to an ischemia-reperfusion injury. This explains the lower graft survival from definite brain damage (DBD) donors as compared to living donors. More so, the DCD donor can aspirate stomach contents in the agonal phase or even postmortem (related to simultaneous abdominal organ recovery). DCD donor assessment is challenging with ethical concerns about possible interventions on a dying patient. Indeed, initial lung assessment may be limited to previous clinical data (with or without bronchoscopic evaluation or even arterial blood gases on standard settings).
These injuries occurring during DCD procurements have profound effects on donor lungs, and there is no way that theses deleterious effects can be assessed before implanting the lung in the recipient chest while using the Standard Care System (SCS) of procurement and preservation on ice. DCD donation was hard to be accepted by most of the lung transplant centers until the EVLP concept proved its safety and evolved. The OCS offers a chance to manage the lungs before implanting them in the recipient, by continuously ventilating and warmly perfusing the lungs before decision is made to use them. It also offers a versatile and easy way of assessing the lungs by monitoring vital parameters, serially checking blood gases and bronchoscopy (which can be done easily through an aperture in the console leading directly to the tracheobronchial tree) while the lungs are fully expanded on the machine.
Use of OCS in brain dead versus cardiac death donor
The steps of utilizing the OCS device for the DCD lungs are different from those related to DBD lungs. With DBD donation, the lungs are accepted following full assessment and ample time to decide to use the OCS machine before aortic cross clamp, which will give enough time to prepare the OCS machine (priming and rewarming) before the donor lung is instrumented and normothermic perfusion process is started (37). For DCD donors, the OCS machine is prepared after organ assessment in the chest. Following the initial flush in the chest of the donor lungs during the procurement process, a visual assessment of the suitability of the donor lungs is performed. Then a decision reached to utilize the OCS device, and next step is the preparation of the machine. This allows the lungs to be stored in cold saline during instrumentation until the machine is ready. In DCD donors, the lungs are not properly recruited before procurement unlike the DBD donor. This opportunity for recruitment is available with OCS which even offers a chance to perform bronchoscopy if it hasn’t been performed yet.
OCS and single lung preservation
OCS is used for bilateral lung preservation according to the company recommendations and FDA approval guidelines. However, in our institution, we used the system in different configurations with good results. In one case the first assessment in donor hospital revealed mild air leak in one lung related to trauma, both lungs were transported on OCS system, the second assessment upon arrival to the recipient hospital revealed that the tear expanded, only the healthy lung was transplanted with excellent outcomes (Belli et al.) (38). The OCS system was also used to perform two single lung transplants in two different recipients. In few cases we kept the second lung warmly perfused and ventilated on OCS system after adjusting those parameters to be suitable for single lung with excellent outcomes. We believe this will keep the second lung in the same physiological status for which the OCS system was intended for use (decrease the cold ischemic time) and give the surgeon more time to transplant the organ. This series of cases are under publication process right now and soon will be published.
LTx and EVLP
The Ex Vivo system (Figure 3) is a static platform that provides a normothermic ventilation-perfusion system that keeps the lungs in a near physiological condition, during which the lungs can be monitored and positively intervened to correct some of the insults that renders the lungs unsuitable for transplantation, such as edema and infection (39).
EVLP technique may help increase the number of the clinically suitable lungs for transplantation by reconditioning those border-lines lungs and give them time to heal before transplanting them. The essential step in lungs’ reconditioning is treating the lung edema that occurs due to the neuro-hormonal imbalance that occur after brain death by perfusing the lungs on the system with a special high-osmolarity solution that helps clear the alveolar space from fluid (40).
The international transplant centers in Sweden and Toronto, who started and promoted this technique, have already published very encouraging results and it became a standard practice in all major centers in the world who adopted this technique after the FDA approved the device.
This system is composed of a ventilator and perfusion circuit. The team conducting this procedure include a surgeon, a respiratory medicine specialist, a perfusionist, and a nurse. This multi−disciplinary team must have extensive education in a realistic simulation environment.
Many clinical devices have evolved since the first systems emerged in Sweden and Toronto. The only FDA approved platform in USA is the XVIVO Perfusion System (XPS) which uses acellular perfusion (Toronto protocol) which has continuously reported good clinical outcomes since 2011 (41).
Principles of Ex Vivo clinical use
The Ex Vivo systems are static platform installed in the recipient hospital. The systems rely either on a cold acellular preservation solution (Acellular technique) that was adopted and modified by Toronto Lung Transplant Institute, or on a low-hematocrit (20%) blood perfusate as originally described by University of Lund, Sweden (Cellular technique). Typically, the donor lung is transported by the procurement team on ice to the recipient hospital where the system installed. The lung is then instrumented on the device and the process of perfusion and ventilation is started. Of note, there is a period of cold ischemia similar to that in the standard care procurement process.
The device has a sterile lung chamber, a centrifugal pump, an oxygenator, a volume reservoir, a heater-cooler system, and a monitor. Perfusion of the lung is performed with an extracellular solution containing buffered dextran. Additionally, the solution has optimized colloid osmotic pressure. The system also uses additives such as Solu-Medrol, antibiotics, and heparin.
Clinical use of EVLP in standard, extended criteria and DCD donors
EVLP is an established clinical approach for evaluation of DCD and Extended Criteria Donors lungs (42). EVLP was developed with the goal of evaluating the quality of those donor lungs before implanting them and to potentially improve the clinical suitability of the lungs for transplant by directly intervene to assess and manage them. Such managements include, in addition to perfusion and ventilation modulation, the use of antibiotics or gene therapy to control infection and enhance the function of these lungs. Initial clinical results have shown promising results, demonstrating that both goals can be achieved, which has expanded the donor organ pool. Indeed, EVLP-treated ECD lungs performed similarly to standard-criteria donor lungs. In addition, EVLP techniques could also play an important role in controlling the times of procurement, assessment, and transplant. These encouraging results in the use of EVLP in ECD donor lungs raises the question for its possible role in standard criteria donor lungs too when time and distance of the donor organ is not within the standard limits.
In 2011, Cypel et al. (39) published in a prospective, nonrandomized clinical trial the results of a large study on the lung features and results of transplantations of ECD lungs (included lung features such as a PaO2:FiO2 <300 mmHg, pulmonary edema, poor compliance, DCD, or massive blood transfusions after EVLP perfusion). It reported that 20 out of the 23 ECD lungs met the endpoint, i.e., PF ratio exceeded 350 mmHg. Furthermore, the study reported that the physiologic parameters on EVLP were within 15% of baseline values, no PGD3 at 72 hours was reported in the recipients of those lungs. The 1-year survival rate between the EVLP patients and the control cohort was 80% and 83.6%, respectively (41). The survival percentages were significantly improved in a follow-up study by the same author, where the reported survival rates for 1-year survival were 87% and the incidence of PGD3 at 72 hours in the EVLP patients who received ECD lungs was 2% (43). Concerning DCD lungs, a report by Machuca et al., used EVLP Toronto protocol to assess 28 donor lungs after DCD procurement (42). They showed the same 1-year survival rates between DCD lungs procured with the use of EVLP and those without the use of EVLP. Additionally, he reported that patients who underwent EVLP LTxs had a 3% incidence of PGD3 at 72 hours following transplantation, while also having a significantly shorter hospital stay (43).
Reports from Sanchez et al. on the ongoing NOVEL trial, which is a prospective, nonrandomized registry of 6 US centers, including ECD lungs placed on EVLP and SCD lungs transplanted without EVLP, preliminary data shows 55% utilization rate with EVLP and a 1-year survival rate similar to that of SCD transplant recipients (44). These results are encouraging and confirm that the use of EVLP in managing and assessing donor lungs in certain situations is very reliable and safe in a well-staffed and equipped transplant center, which until short time ago deemed unsafe to approach. Its use will give a noticeable increase in the number of lung transplants. Although these results are encouraging, we are still unable to assess the long follow up outcomes.
Conclusions
With the increased number of patients waiting for lung transplant, we are in constant need to expand the pool of suitable donor lungs. In order to achieve this goal, we might need to think outside of the box to revise our definition and assessment criteria by which we judge the “transplantability” of those organs. One of these ways is to broaden the donor pool by accepting more “borderline” lungs from outside standard criteria by widening donor age, smoking restrictions, lung trauma level, bronchial secretions, and travel distance (referring to ischemic time). In order to reach this goal, we need also reproducible safe clinical tools to procure, manage, and assess those lungs before they are successfully transplanted. Expanding the donor pool could also be achieved by accepting more organs from DCD. Finally supported by the promising results of the clinical trials of using EVLP systems, whether static (Ex Vivo) or mobile (OCS), to assess and manage those lungs for safe clinical use and successful transplant is essential. All these measures likely will lead to an increase in the number of suitable donor lungs in the future.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (George Makdisi) for the series “Lung Transplant: Current Status and Challenges” published in Current Challenges in Thoracic Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://ccts.amegroups.com/article/view/10.21037/ccts-21-25/rc
Peer Review File: Available at https://ccts.amegroups.com/article/view/10.21037/ccts-21-25/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts-21-25/coif). The series “Lung Transplant: Current Status and Challenges” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Valapour M, Lehr CJ, Skeans MA, et al. OPTN/SRTR 2017 Annual Data Report: Lung. Am J Transplant 2019;19:404-84. [Crossref] [PubMed]
- Botha P, Rostron AJ, Fisher AJ, et al. Current strategies in donor selection and management. Semin Thorac Cardiovasc Surg 2008;20:143-51. [Crossref] [PubMed]
- Fischer S, Gohrbandt B, Struckmeier P, et al. Lung transplantation with lungs from donors fifty years of age and older. J Thorac Cardiovasc Surg 2005;129:919-25. [Crossref] [PubMed]
- Aigner C, Winkler G, Jaksch P, et al. Extended donor criteria for lung transplantation--a clinical reality. Eur J Cardiothorac Surg 2005;27:757-61. [Crossref] [PubMed]
- Demikhov VP. Experimental Transplantation of Vital Organs. New York: Consultants Bureau, 1962.
- Wildevuur CR, Benfield JR. A review of 23 human lung transplantations by 20 surgeons. Ann Thorac Surg 1970;9:489-515. [Crossref] [PubMed]
- Hardy JD, Erasian S, Dalton ML. Autotransplantation and homotransplantation of the lung: Further studied. J Thorac Cardiovasc Surg 1953;46:606. [Crossref] [PubMed]
- Toronto Lung Transplant Group. Unilateral lung transplantation for pulmonary fibrosis. N Engl J Med 1986;314:1140-5. [Crossref] [PubMed]
- Egan TM, Murray S, Bustami RT, et al. Development of the new lung allocation system in the United States. Am J Transplant 2006;6:1212-27. [Crossref] [PubMed]
- Egan TM, Edwards LB. Effect of the lung allocation score on lung transplantation in the United States. J Heart Lung Transplant 2016;35:433-9. [Crossref] [PubMed]
- Sade RM. Brain death, cardiac death, and the dead donor rule. J S C Med Assoc 2011;107:146-9. [PubMed]
- Manara AR, Murphy PG, O'Callaghan G. Donation after circulatory death. Br J Anaesth 2012;108:i108-21. [Crossref] [PubMed]
- Van Raemdonck D, Keshavjee S, Levvey B, et al. Donation after circulatory death in lung transplantation-five-year follow-up from ISHLT Registry. J Heart Lung Transplant 2019;38:1235-45. [Crossref] [PubMed]
- Valenza F, Citerio G, Palleschi A, et al. Successful Transplantation of Lungs From an Uncontrolled Donor After Circulatory Death Preserved In Situ by Alveolar Recruitment Maneuvers and Assessed by Ex Vivo Lung Perfusion. Am J Transplant 2016;16:1312-8. [Crossref] [PubMed]
- Hardy JD, Webb WR, Dalton ML Jr, et al. Lung homotransplantation in man. JAMA 1963;186:1065-74. [Crossref] [PubMed]
- D'Alessandro AM, Hoffmann RM, Knechtle SJ, et al. Controlled non-heart-beating donors: a potential source of extrarenal organs. Transplant Proc 1995;27:707-9. [PubMed]
- Kootstra G, Daemen JH, Oomen AP. Categories of non-heart-beating donors. Transplant Proc 1995;27:2893-4. [PubMed]
- Daemen JW, Kootstra G, Wijnen RM, et al. Nonheart-beating donors: the Maastricht experience. Clin Transpl 1994;303-16. [PubMed]
- Egan TM, Lambert CJ Jr, Reddick R, et al. A strategy to increase the donor pool: use of cadaver lungs for transplantation. Ann Thorac Surg 1991;52:1113-20; discussion 1120-1. [Crossref] [PubMed]
- Oto T, Levvey B, McEgan R, et al. A practical approach to clinical lung transplantation from a Maastricht Category III donor with cardiac death. J Heart Lung Transplant 2007;26:196-9. [Crossref] [PubMed]
- Thuong M, Ruiz A, Evrard P, et al. New classification of donation after circulatory death donors definitions and terminology. Transpl Int 2016;29:749-59. [Crossref] [PubMed]
- Gomez-de-Antonio D, Campo-Cañaveral JL, Crowley S, et al. Clinical lung transplantation from uncontrolled non-heart-beating donors revisited. J Heart Lung Transplant 2012;31:349-53. [Crossref] [PubMed]
- Egan TM, Haithcock BE, Lobo J, et al. Donation after circulatory death donors in lung transplantation. J Thorac Dis 2021;13:6536-49. [Crossref] [PubMed]
- Nuñez JR, Varela A, del Río F, et al. Bipulmonary transplants with lungs obtained from two non-heart-beating donors who died out of hospital. J Thorac Cardiovasc Surg 2004;127:297-9. [Crossref] [PubMed]
- Levvey BJ, Westall GP, Kotsimbos T, et al. Definitions of warm ischemic time when using controlled donation after cardiac death lung donors. Transplantation 2008;86:1702-6. [Crossref] [PubMed]
- Steen S, Sjöberg T, Pierre L, et al. Transplantation of lungs from a non-heart-beating donor. Lancet 2001;357:825-9. [Crossref] [PubMed]
- D'Alessandro AM, Fernandez LA, Chin LT, et al. Donation after cardiac death: the University of Wisconsin experience. Ann Transplant 2004;9:68-71. [PubMed]
- Mason DP, Murthy SC, Gonzalez-Stawinski GV, et al. Early experience with lung transplantation using donors after cardiac death. J Heart Lung Transplant 2008;27:561-3. [Crossref] [PubMed]
- The U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients. 2009 OPTN/SRTR annual report. Available online: http://optn.transplant.hrsa.gov/ar2009/
- Popov AF, García Sáez D, Sabashnikov A, et al. Utilization of the organ care system - a game-changer in combating donor organ shortage. Med Sci Monit Basic Res 2015;21:29-32. [Crossref] [PubMed]
- Wang X, O'Brien ME, Yu J, et al. Prolonged Cold Ischemia Induces Necroptotic Cell Death in Ischemia-Reperfusion Injury and Contributes to Primary Graft Dysfunction after Lung Transplantation. Am J Respir Cell Mol Biol 2019;61:244-56. [Crossref] [PubMed]
- Whitson BA, Prekker ME, Herrington CS, et al. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J Heart Lung Transplant 2007;26:1004-11. [Crossref] [PubMed]
- Warnecke G, Van Raemdonck D, Smith MA, et al. Normothermic ex-vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE): a randomised, open-label, non-inferiority, phase 3 study. Lancet Respir Med 2018;6:357-67. [Crossref] [PubMed]
- Loor G, Warnecke G, Villavicencio MA, et al. Portable normothermic ex-vivo lung perfusion, ventilation, and functional assessment with the Organ Care System on donor lung use for transplantation from extended-criteria donors (EXPAND): a single-arm, pivotal trial. Lancet Respir Med 2019;7:975-84. [Crossref] [PubMed]
- Loor G. EVLP: Ready for Prime Time? Semin Thorac Cardiovasc Surg 2019;31:1-6. [Crossref] [PubMed]
- Yusen RD, Edwards LB, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Lung and Heart-Lung Transplant Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant. J Heart Lung Transplant 2016;35:1170-84. [Crossref] [PubMed]
- Wiegmann B, Falk CS, Muller B, et al. Effect of Warm Perfusion of Donor Lungs Using the OCS on Immune Mediator Release during Preservation. J Heart Lung Transplant 2013;32: [Crossref]
- Belli EV, Katlaps G, Al Salihi M, et al. Use of Organ Care System Lung for Single-Lung Transplantation. Ann Thorac Surg 2021;111:e43-4. [Crossref] [PubMed]
- Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med 2011;364:1431-40. [Crossref] [PubMed]
- Tane S, Noda K, Shigemura N. Ex Vivo Lung Perfusion: A Key Tool for Translational Science in the Lungs. Chest 2017;151:1220-8. [Crossref] [PubMed]
- Cypel M, Yeung JC, Machuca T, et al. Experience with the first 50 ex vivo lung perfusions in clinical transplantation. J Thorac Cardiovasc Surg 2012;144:1200-6. [Crossref] [PubMed]
- Machuca TN, Mercier O, Collaud S, et al. Lung transplantation with donation after circulatory determination of death donors and the impact of ex vivo lung perfusion. Am J Transplant 2015;15:993-1002. [Crossref] [PubMed]
- Sage E, Mussot S, Trebbia G, et al. Lung transplantation from initially rejected donors after ex vivo lung reconditioning: the French experience. Eur J Cardiothorac Surg 2014;46:794-9. [Crossref] [PubMed]
- Sanchez PG, Davis RD, D'Ovidio F, et al. The NOVEL lung trial one-year outcomes. J Heart Lung Transplant 2014;33:S71-S72. [Crossref]
Cite this article as: Al Salihi M, Alsalihi Y, Cahill J. Boosting lung transplantation, a narrative review of the impact of donor after cardiac death, organ care system and ex vivo lung perfusion in expanding donor pool. Curr Chall Thorac Surg 2023;5:19.