
Robotic right upper lobe segmentectomy
Introduction
Anatomic segmentectomy for early-stage non-small cell lung cancer (lesions ≤2 cm), has been shown to achieve similar oncologic outcomes to lobectomy while preserving lung function (1). Segmentectomy requires preoperative planning and intraoperative dexterity to anticipate variations in anatomy and approach. Robotic segmentectomy has been shown to be safe with excellent perioperative outcomes, which is aided by the use of infrared contrast for nodule identification and delineation of the intersegmental plane (2). The use of the robotic platform for segmentectomy has increased by over 500% over the last ten years, with over 800 robotic segmentectomies performed in 2018 (3).
The most commonly performed segments in the right upper lobe (RUL) are the posterior (S2), apicoposterior (S1, S2), anterior (S3), and then apical (S1) segment. While the apicoposterior (S1, S2) segment can be performed, that resection in the RUL leaves only a single anterior segment, as opposed to for the left upper lobe which leaves the anterior segment along with the lingular segments. Additionally, if the patient has a complete minor fissure, the remaining anterior segment is prone to torsion. Thus, the authors do not recommend apicoposterior (S1, S2) segmentectomy in the RUL, and it will not be described in this article. For the remaining isolated segmentectomies, each segment has its particular strategy and exposures for resection of the corresponding segmental artery, vein and bronchus. In all cases, we recommend a complete lymph node dissection, including the interlobar lymph nodes (station 11).
RUL anatomy
The right lung has three lobes: the upper lobe, the middle lobe, and the lower lobe. The major (oblique) fissure divides the upper and middle lobes from the lower lobe, while the minor (horizontal) fissure separates the upper lobe from the middle lobe. The RUL is comprised of three segments: the apical (S1), posterior (S2), and anterior (S3) segments. Each segment is comprised of its own bronchus, pulmonary arterial supply, and pulmonary venous drainage.
Bronchial anatomy
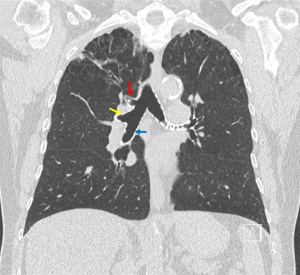
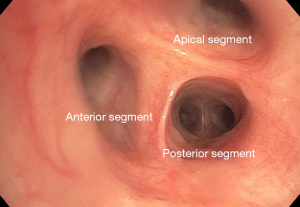
The right mainstem bronchus bifurcates into the RUL bronchus and the bronchus intermedius. Rarely, in less than 0.5% of patients, the RUL bronchus originates from the trachea, proximal to the carina (4). Even more rare is a “porcine” bronchus when the apical bronchus arises from the distal trachea, but the remaining upper lobe bronchi arise from the standard position off the right mainstem bronchus (Figure 1). The RUL bronchus trifurcates into the apical (B1), posterior (B2), and anterior (B3) bronchi. While there is normally a trifurcation (Figure 2), other anatomic variants can exist, such as two separate bifurcations dividing into three segmental bronchi (5).
Arterial anatomy
The pulmonary artery (PA) divides into the right PA and left PA. The right PA runs inferior/anterior to the bronchus, and superior/posterior to the pulmonary vein (PV) branches. The first branch from the right PA is the truncus anterior, which arises from the superior aspect of the right PA. The truncus anterior (A1, A3) often branches into two segments which supplies the apical segment (S1) and anterior segment (S3). However, A1 and A3 can also arise from two separate branches of the right PA. Another branch of the PA, the posterior ascending (A2), often arises from the interlobar PA and goes to the posterior segment (S2). Many variants can occur, including the entire lobar blood supply arising from the truncus anterior, multiple posterior ascending branches, the A2 branch arising off the superior segment PA (A6) or middle lobe PA (A4, A5), or the A6 branch arising from A2 (5).
Venous anatomy
The PV vein branches are the most anterior hilar structure. The superior PV often drains the RUL and right middle lobe, and lies anterior to the PA. The RUL PV often split into the apical anterior (V1), anteroinferior (V3), and posterior (V2) vein branches. Similar to the PA, V2 can drain into the superior segment vein (V6) or V6 may drain into V2. Rarely, V3 may run posterior to the bronchus intermedius, or the upper lobe vein can drain into the azygous vein or the superior vena cava (SVC).
Robotic RUL segmentectomy
Port placement
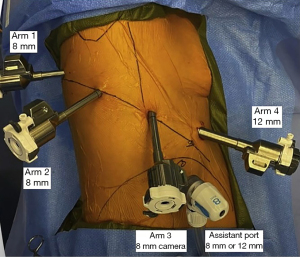
Proper placement of the robotic ports is crucial to setting up and successfully performing any robotic pulmonary resection. Our institution uses a da Vinci Xi system (Intuitive Surgical, Sunnyvale, CA, USA), with four robotic ports and an assistant port. After left lateral decubitus positioning with a double lumen endotracheal tube for single lung ventilation, the patient is prepped and draped. The scapula is marked, and a line is drawn along the vertebral right lateral pedicles. The ribs are palpated and counted to identify to the 9th rib. Incision sites for robotic arms 1, 2, and 3 (all 8 mm ports) are marked in the 8th interspace along the line of the 9th rib, measured from its intersection with the right lateral pedicle. Robotic arm 1 is drawn 4 cm from the right lateral pedicle, arm 2 is drawn 8 cm medial to robotic arm 1, and the camera port (robotic arm 3) is drawn 8 cm medial to robotic arm 2. Occasionally, a smaller patient size may require shorter distances, such as 2 cm, 7 cm, and 7 cm, respectively.
An incision is made at the camera port site marking, and the pleural space is entered bluntly with a clamp. An 8 mm trocar is placed, then camera is inserted to ensure entry into the thoracic cavity prior to insufflation. Under thoracoscopic camera guidance, analgesia is administered via subpleural injection of local anesthetic at the remaining planned port sites. Concurrently, an intercostal paraspinal block is performed. The remaining 8 mm ports are placed under camera guidance. The final robotic port is a 12 mm port for arm 4, and is placed anteriorly in the 7th interspace just above the diaphragm. The 7th interspace is used to avoid the rectus muscles. The assistant port (8 mm or 12 mm if using a handheld stapler) is placed between the camera port and the port for robotic arm 4, just superior to the diaphragm (Figure 3).
Instruments
After the robot is docked, the following instruments are used throughout the case:
- Robotic arm 1—tips up grasper;
- Robotic arm 2—Cadiere forceps;
- Robotic arm 3—0 degree robotic camera;
- Robotic arm 4—long bipolar grasper (with energy). This port is also used for robotic staplers.
Mediastinal lymph node dissection
Once all instruments are in, we first proceed with a complete mediastinal lymph node dissection. The right lower lobe is retracted up, and the inferior pulmonary ligament is divided. All level 8 and 9 lymph nodes are removed. The lung is then retracted anterior, and pleura overlying the posterior hilum is divided. The level 7 subcarinal lymph node packet is completely dissected, with care to ensure hemostasis. Next, the interlobar lymph node (level 11R) present at the division of the RUL bronchus and right bronchus intermedius is carefully dissected and removed, revealing the posterior ascending (A2) and superior segment (A6) arteries. The lung is then retracted inferiorly to expose the azygous vein. The pleura between the SVC and trachea is opened, and the level 2R and 4R lymph node packets are carefully dissected out, with care to avoid the vagus nerve. During this time, the hilar lymph nodes (level 10 R) can be dissected off the superior aspect of the PA, which may ease dissection of the segmental PA branches.
Posterior segmentectomy (S2)
The most common segmental resection performed in the RUL is a posterior segmentectomy (S2). An S2 resection is performed from a posterior approach. After the mediastinal lymph node dissection, the lung is retracted anteriorly. The posterior ascending PA (A2) is identified in the posterior fissure, with the V2 vein coursing above the A2 branch (Figure 4). Usually the posterior fissure can then be completed. If the fissure is difficult to complete, you can divide the A2 and posterior bronchus (B2) first. Otherwise, after division of the posterior fissure, the A2 branch is carefully dissected to avoid injury to V2, and divided with a robotic stapler. Next, the V2 vein is divided with a robotic stapler. Afterward, B2 is visible superiorly. Care must be taken during dissection of B2 to not injure the PA branches arising off the truncus anterior (A1, A3), which lie anterior to the RUL bronchus. All lobar and segmental lymph nodes are removed (level 12R and 13R) throughout the segmental resection.
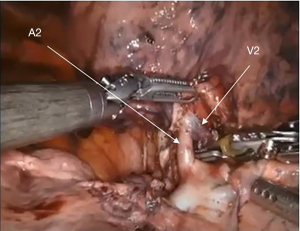
Common pitfalls to avoid are injury to V2 while dissecting A2, as well as injury to A1, A3 while dissecting out B2. Anatomic variants that need to be carefully evaluated are related to the vascular structures. A2 can arise off A6 or A6 can arise off the A2 branch. In either case, the anatomy must be clearly delineated to avoid injury to or accidental division of A6. Similar anatomic variants occur with the venous structures, where V2 can drain into the superior segment PV (V6) or vice versa. Again, care must be taken to identify these anomalies to avoid division or injury of V6, which can lead to segmental necrosis.
Anterior segmentectomy (S3)
A segmental resection of the anterior segment (S3) is less commonly performed than an S2 resection. After the mediastinal lymph node dissection, the lung is retracted posteriorly, and the pleura overlying the anterior hilum is dissected, with care to preserve the phrenic nerve. The superior PV is carefully dissected, and retracted inferiorly with a vessel loop. Next, the anterior vein (V3), which is the most anterior segmental venous branch, should be dissected and divide, though there is risk of injury to the PA when passing the stapler to divide V3. Then, the truncus anterior (A1, A3) is dissected. Throughout the resection, all 12R and 13R lymph nodes are removed. The A3 branch is carefully encircled and divided. The minor fissure can be completed with a stapler, with care to stay anterior to the V2 branch. Underneath the divided A3, the V2 branch is adjacent to the anterior bronchus (B3), and courses between B2 and B3. B3 is encircled, with care to avoid injury to the V2, as well as the apical PA (A1). B3 can be divided with a robotic stapler.
Apical segmentectomy (S1)
An apical segmental (S1) resection is rarely performed, but is performed from an anterior or a superior approach. After dissection of the mediastinal lymph nodes, the lung is reflected posteriorly and the anterior pleura is opened from the base of the superior PV to the apex of the hilum, with care to preserve the phrenic nerve. The apical vein (V1) and artery (A1) are identified and dissected. V1 is then encircled and divided. A1 is then divided. Directly under the divided artery is the apical bronchus (B1), which is best exposed by lifting up the lung parenchyma, and dissecting out the proximal three branches of B1, B2, and B3 and removing the intersegmental lymph nodes. B1 is encircled and stapled, with care to avoid injury to B3. Similar to all segmental resection, all 12R and 13R lymph nodes are removed as part of the dissection.
Division of the segmental fissure
After the segmental structures are divided, indocyanine green is injected intravenously. Using firefly on the robotic camera, the intersegmental plane is identified and marked with cautery. Robotic or bedside staplers are used to complete the parenchymal fissure, with care to include the segmental structures in the specimen (Table 1).
Table 1
| Right upper lobe segment | Approach | Steps | Common pitfalls |
|---|---|---|---|
| Posterior segment (S2): most common right upper lobe segment | Posterior hilar dissection | Identify the A2 in the fissure | Injury to V2 while dissecting A2 |
| Identify V2 and A6 | Injury to A1, A3 while dissecting B2 | ||
| Open the posterior fissure | A2 arises from A6 or A6 arises from A2 | ||
| Divide A2 | V2 drains into V6 or V6 drains into V2 | ||
| Divide V2 | |||
| Dissect out the bronchus (B2), with care to avoid injury to A1, A3 | |||
| ICG injection and parenchymal division | |||
| Anterior segment (S3): infrequently performed | Anterior hilar dissection | Dissect the superior pulmonary vein | Injury to A1, A3 while dissecting and stapling V3 |
| Must visualize and protect the phrenic nerve | Divide V3 at this time point | Division or injury to V2 while opening the minor fissure | |
| Retract superior pulmonary vein inferiorly and expose A1, A3 | Injury to A1 or V2 when dissecting B3 | ||
| Dissect and staple A3 | |||
| Complete the minor fissure, staying anterior to V2 | |||
| Encircle B3, with care to not injure A1 or V2 | |||
| Divide B3 | |||
| ICG injection and parenchymal division | |||
| Apical segment (S1): rarely performed | Anterior hilar dissection | Dissect and identify A1 and V1 | Injury A3 when dividing V1 |
| Must visualize and protect the phrenic nerve | Divide V1 | Injury to A3 when dividing B1 | |
| Divide A1 at this time point | |||
| Lift lung parenchyma to expose B1 underneath A1 | |||
| Dissect and divide B1 | |||
| ICG injection and parenchymal division |
A1, apical pulmonary artery; A2, posterior pulmonary artery; A3, anterior pulmonary artery; A6, superior segment pulmonary artery; V1, apical pulmonary vein; V2, posterior pulmonary vein; V3, anterior pulmonary vein; V6, superior segment pulmonary vein; B1, apical bronchus; B2, posterior bronchus; B3, anterior bronchus. ICG, indocyanine green.
Closure
A specimen bag is placed through the anterior port (arm 4), with the use of robotic arm 1 to pull the corner of the bag inferiorly, aiding in deploying the bag with a large opening. The specimen is placed in the bag. Prior to removing the specimen, the bag is examined to ensure that it is not caught on any staple lines, which can result in significant bleeding when pulling out the bag. After removal of the segment, hemostasis is obtained, and a 20 Fr chest tube is placed apically. The lung is re-expanded under direct visualization, with care to ensure the remaining RUL and the right middle lobe are not torsed.
Postoperative care
Patients undergoing robotic segmentectomy are treated with multimodality pain control at three phases of care: preoperatively with acetaminophen and gabapentin, intraoperatively with an intercostal and field block using local anesthetic, and postoperatively with a combination of acetaminophen, gabapentin, and opioids as needed. A single chest tube is placed post resection. If no air-leak is present with a fully expanded lung on postoperative chest radiograph, the patient’s chest tube is removed after 6–8 hours, regardless of quantity of fluid output. For these patients, they are given a fatty meal to assess for chylothorax, and if none is present, the tube is removed. Patients with an air-leak are connected to a digital chest drainage system to allow ambulation and accurate assessment of air-leak quantity and/or cessation. The majority of patients are able to discharge on postoperative day number one after robotic segmentectomy.
Conclusions
With a complete mediastinal lymph node dissection, as well as a lobar and segmental lymph node removal, segmental resection of the RUL remains a good parenchymal-sparing resection for select small pulmonary lesions. The robotic platform is a useful approach to performing RUL segmentectomies. However, each segment has possible anatomic variants or common pitfalls that each surgeon should be cognizant of during the procedure.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Richard Lazzaro) for the series “Robotic Anatomic Pulmonary Resection” published in Current Challenges in Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts-20-177/coif). The series “Robotic Anatomic Pulmonary Resection” was commissioned by the editorial office without any funding or sponsorship. MZ reports a financial relationship with Intuitive Surgical Inc. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Geraci TC, Ferrari-Light D, Kent A, et al. Technique, Outcomes With Navigational Bronchoscopy Using Indocyanine Green for Robotic Segmentectomy. Ann Thorac Surg 2019;108:363-9. [Crossref] [PubMed]
- Servais EL, Towe CW, Brown LM, et al. The Society of Thoracic Surgeons General Thoracic Surgery Database: 2020 Update on Outcomes and Research. Ann Thorac Surg 2020;110:768-75. [Crossref] [PubMed]
- Doolittle AM, Mair EA. Tracheal bronchus: classification, endoscopic analysis, and airway management. Otolaryngol Head Neck Surg 2002;126:240-3. [Crossref] [PubMed]
- Veeramachaneni NK. Surgical anatomy of the Lungs. Shield’s General Thoracic Surgery. 8th edition; 2018.
Cite this article as: Chang SH, Geraci TC, Kent AJ, Pass H, Bizekis C, Zervos M, Cerfolio RJ. Robotic right upper lobe segmentectomy. Curr Chall Thorac Surg 2023;5:22.


