A narrative review of the social determinants of lung cancer screening: knowledge gaps and controversies
Introduction
Lung cancer is the second most prevalent malignancy and most common cause of cancer-related death worldwide (1). While the utility of routine lung cancer screening (LCS) with low-dose computed tomography (LDCT) had previously been under debate, its mortality benefit is now established after reports from multiple prospective trials (2,3). In response, there has been an expansion of LCS programs for asymptomatic, high-risk individuals, with downstream increases in diagnosis of early-stage disease and long-term survival (4,5). However, these reassuring metrics are accompanied by limitations in screening practices, especially when applying LCS recommendations toward marginalized groups (6,7). Historically, cancer screening guidelines are drawn from clinical trials that largely underrepresent minorities and women (8). This has fostered further discussion on screening strategies and protocols, exposing a need for strategic screening practices for marginalized groups and minority populations. The aim of the current narrative review is to present these knowledge gaps and controversies, with a focus towards disparities and social determinants associated with lung cancer management. Given the juxtaposition of the relative homogeneity of large LCS trials and the heterogeneity of LCS in clinical practice, the authors of the current review aim to clarify areas of consideration for more generalizable LCS practices. We present this article in accordance with the Narrative Review reporting checklist (available at https://ccts.amegroups.com/article/view/10.21037/ccts-22-4/rc).
Methods
PubMed was searched for cancer screening trial designs, with LCS disparities as the focus, from years 2000 to 2023. The keywords specifically included “cancer screening, lung cancer screening, trial design, health equity, disparities, racial, ethnicity, socioeconomic”. Given the breadth of health disparities research in lung cancer screening, keywords searched were limited to these more focused terms. Randomized trials, retrospective cohort and cross-sectional studies, and systematic reviews were included (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | January 6th, 2022; May 25th, 2023 |
| Databases and other sources searched | PubMed |
| Search terms used | The following terms have been used: lung cancer disparities, lung cancer screening disparities, lung cancer social determinants, lung cancer inequity, lung cancer risk, barriers to lung cancer, barriers to lung cancer screening, lung cancer trial design, lung cancer health equity, lung cancer racial, lung cancer ethnicity, lung cancer African American, lung cancer Black, lung cancer Hispanic, lung cancer Asian, lung cancer socioeconomic status, lung cancer insurance status |
| Timeframe | Between January 1st, 2000 and May 25th, 2023 |
| Inclusion and exclusion criteria | Inclusion criteria: systematic reviews, randomized clinical trials, retrospective and cross-sectional studies, meta-analyses and CS guidelines |
| Exclusion criteria: publications considered were restricted to those published in English language | |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | SM Adnan conducted the selection process. K Chin reviewed the selected papers and added additional references that may have been missed |
CS, cancer screening.
Disparities stemming from randomized trial design
The largest LCS trials report a mortality benefit of routine screening with LDCT, at least for high-risk populations. The United States National Lung Screening Trial (NLST) included current and former heavy smokers who were 55–74 years in age, which subsequently resulted in the US Preventative Services Task Force (USPSTF) recommendation for annual screening with LDCT for this population (2). Similar inclusion criteria were utilized in the Nederlands-Leuvens Longkanker Screenings Onderzoek (NELSON) trial, with screening practices aimed towards this demographic since (3). However, the homogeneity of these “high-risk” populations may limit more generalizable and granular conclusions, largely due to incomplete representation of minority and underserved groups. Specifically, the NLST cohort consisted of roughly 90% non-Hispanic White participants, and while the NELSON trial did not comment on participant race, the demography of the included countries was roughly 95% non-Hispanic White.
Within the context of this narrative review, health disparity must be explicitly differentiated from health inequity, given that ambiguity in these definitions may confound interpretation and future interventions. Health disparities, in this context will refer to the status of unequal health potential whereas health inequity is a difference in individuals’ opportunity to attain the highest level of health (9).
The lack of diversity in the aforementioned landmark trials manifests as a health disparity. For instance, Black and African American men are diagnosed with lung cancer at earlier ages and with lesser smoking histories (10). Unfortunately, these high-risk individuals are oftentimes ineligible for screening based on USPSTF criteria (10). Large population-based studies further carry inherent biases, with several methodological challenges that perpetuate health inequity (11). These limitations of landmark trials manifest as restrictive screening recommendations, which preclude some high-risk, marginalized groups from the life-saving benefits of LCS. However, broadening screening recommendations must be balanced against the potential harms of overdiagnosis and false positives, perhaps by more selective criteria for screen-indeterminate candidates.
Other areas of homogeneity in cancer screening trials further limit generalizability. Most participants in cancer trials are younger and with fewer comorbidities. They also have higher rates of health literacy and college education, suggesting an inherent bias in the form of a “healthy volunteer effect” (12). These trials are usually conducted at tertiary care centers, which may overlook community practice standards and rural populations with more limited access to care. The lack of diversity in cancer trial design warrants attention, especially when tailoring LCS guidelines towards marginalized groups. More targeted data is needed among populations based on race, ethnicity, geography and other social determinants of health including insurance status and access to screening and multidisciplinary care.
Marginalized groups in LCS
The underrepresentation of minorities and marginalized groups in LCS trials has resulted in more restrictive criteria for screening eligibility. Even when accounting for the biases observed in LCS trial design, only 3.3% to 20% of screen-eligible individuals ultimately participate in LCS (13). This uptake rate is even lower in African American and Hispanic communities, which have up to 50% lower rates of screening in comparison to non-Hispanic Whites (10). A similar trend is also observed in those with greater socioeconomic barriers (14,15). This inequity warrants specific attention for developing more equitable LCS guidelines and management protocols. The low uptake rate of screen-eligible candidates is likely a result of interacting factors, such as awareness of screening benefits, availability, and perceived barriers to screening. This critical issue must first be addressed by understanding disparities in LCS, with a subsequent focus on outreach measures for at-risk populations.
Racial disparities: Black and African Americans
Black and African Americans are largely underrepresented in cancer screening trials, even though secondary analysis of the NLST suggests that they experience the greatest relative reduction in lung cancer-related mortality with LDCT screening (16,17). However, demographic factors linked with improved survival (i.e., higher education, fewer comorbidities, former smoking status), are less observed within this minority group (15). This manifests as an inequity, given that Black and African Americans have lower rates in screening participation, with poorer adherence to follow-up screening and treatment recommendations. Partly to address this inequity, the USPSTF revised LCS recommendations, decreasing the age of eligibility from 55 to 50 years, and smoking pack-years from 30 to 20 (18). When looking retrospectively at a diverse population, these new criteria improved the ability of LCS to detect lung cancer among African Americans (19). While expansion of LCS screening criteria is encouraging, screening based strictly on age and smoking history overlooks more complex, influential racioethnic and socioeconomic differences.
Screening can only improve survival if detected cancers are treated, a process that requires several follow-up visits, further diagnostic testing, and eventual treatment. When controlling for all other social determinants of health variables, self-reported Black race was associated with 1.5 times the odds of missing LCS appointments compared to self-reported White race (20). Follow-up in the NLST was roughly 95%, though a prospective study of an underserved population reported only an 18.7% adherence to annual screening upon follow-up (21). This marked difference implies additional barriers for this minority group, consistent with other reports that Black and African Americans are less likely to undergo LCS and receive first-line therapy (22-24). This marginalized group is also noted to have more limited knowledge of lung cancer and the benefits of screening (25). Black and African Americans are less likely to engage in research initiatives that shape screening protocols, partly due to patient-related factors inherent to underserved minority communities (i.e., health literacy, cost concerns, insurance coverage). The unawareness of the benefits of screening is compounded by competing health issues and barriers to care access. The challenges Black and African American communities face are multifactorial, stemming from underrepresentation from LCS trials and extending into community inequities, which together warrant a focused approach to reduce these health disparities.
Socioeconomic status (SES) and insurance disparities
Lower SES results in a disparity in incidence and mortality of lung cancer, more so than any other cancer type (26). This may be due to higher rates of smoking, earlier smoking debut, and overall greater pack-years consumed by lower SES groups (27). Lower SES neighborhoods also seem to be more targeted by retailers and advertisements from the tobacco industry, which may partly contribute to lower screening rates, increased diagnosis at later-stage disease, and lower rates of surgical resection for early-stage disease observed in these communities (28). Regardless of such reports, higher SES groups are still over-represented in LCS studies (29).
The socioeconomic barriers to LCS are undoubtedly complex and multifactorial, and current LCS guidelines rely on prospective trials that focus on a high-risk, homogenous cohort. With the emergence of health disparities research, the interaction between SES and LCS participation is now well-documented (30,31). A recent analysis of LCS rates at an urban, safety-net hospital reported a lower median household income for unscreened patients. This report was followed by a more targeted statement that current LCS protocols overlook socioeconomic factors in lung cancer risk (32). For instance, lower-income patients may forego screening if they believe there are transportation barriers to screening centers, or if the financial cost of screening outweighs supposed benefits. They may also undergo screening and decline therapy due to other economic and logistical barriers, thereby incurring the harms of screening without experiencing any of the benefits. These barriers should be acknowledged when developing an effective, generalized screening program, which should be more purposeful in management delivery. For instance, patient reminders in the form of periodical letters and brochures have been shown to increase screening rates for other malignancies (33). One-to-one education that highlights the LCS process, with an emphasis on the risks and benefits of radiological screening for early-stage disease, may increase screening participation.
Geographical barriers should also be addressed, either in the form of more available transportation services or establishment of more widespread screening facilities, both of which are lacking in states with the greatest incidence of and mortality related to lung cancer (34). In a study of the 2015 National Health Interview Survey by Odahowski et al., LCS was widely underutilized in both metropolitan and nonmetropolitan areas (3.83% and 3.72%, respectively), but 23% of nonmetropolitan people were eligible for LCS despite comprising only 15% of the U.S. population (35). This compounds upon other reports suggesting that LCS screening programs are not distributed according to need. In a study of rural Missouri, 41% of nonmetropolitan residents lived more than 30 miles away from a LCS screening center, and the odds of having access to screening in rural areas were 17% of the odds in metropolitan areas (36).
A similar disparity is seen with insurance status, which is closely linked with SES. Bradley et al. reported nearly a 5-fold increased incidence of lung cancer in younger (<65 years), Medicaid-eligible patients through retrospective analysis of a statewide cancer registry (37). In the aforementioned study, older individuals on Medicaid were also reported to have a higher incidence of lung cancer, in comparison to those with private insurance. A separate retrospective analysis of reported an association of 6.1 times the odds of missing a scheduled LCS appointment for patients on Medicaid insurance (20). This association extended to lower SES groups, which was associated with dual eligibility for Medicare and Medicaid. A retrospective cohort study of 2,626 older individuals with early-stage lung cancer demonstrated that those with dual eligibility were less likely to undergo surgical resection than Medicare-only patients. Moreover, those who did undergo resection had greater overall mortality (38). Even for screen-detected early-stage disease, lower SES groups (including those on Medicaid or without insurance) were more likely to receive no treatment or nonstandard treatment. This suggests that while government insurance may relieve some financial constraints faced by lower SES groups, other existing barriers limit access to quality screening and management.
More importantly, current Medicare eligibility for LCS may not align with lung cancer risk in racial minorities. Black and Hispanic Americans are more likely to be current smokers but have fewer total pack-years than non-Hispanic Whites. When considering patients who fall within the Medicare eligibility criteria for LCS, African American men were found to have a higher risk of developing lung cancer in comparison to non-Hispanic White men, with the inverse being true for Hispanic men (39). This suggests the current state of underscreening and over-screening for lung cancer in African American and Hispanic populations, respectively, with targeted attention warranted towards the nuances of risk in these populations. A risk-prediction model derived from the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCOM2012) demonstrated a 12.4% increased LCS eligibility for African American men and a 10.8% decreased LCS eligibility for Hispanic men, reinforcing the sensitivity of a more granular risk-based model in regards to race (40). This highlights the need for more specialized screening practices, especially given the lower rates of treatment, larger delays to treatment, and pathological upstaging experienced by these minority groups (41). In addition to age and smoking history, integration of gender, race/ethnicity, exposure history, comorbidities, and family history may provide a more comprehensive assessment of individual risk.
Complexities of racial minority groups: paradoxical associations
With the aforementioned health disparities noted, there are other social determinants towards LCS without concrete explanations, some of which may appear paradoxical. Similar to Black and African Americans, Hispanic groups are more likely to be uninsured, have a lower SES, and experience treatment delays for lung cancer (42). However, they experience a survival advantage in comparison to non-Hispanic Whites, despite an overall greater risk profile (43). This may be explained by cultural factors that facilitate social integration, largely the impact of family and collectivism, which may influence overall resilience in the post-diagnosis stage for lung cancer management. Further research is needed to clarify this paradoxical survival benefit observed in the Hispanic community, given the several other health disparities they encounter.
Asian communities are largely underrepresented in LCS trials and exhibit unique paradoxical relationships as well. For instance, when considering women with no smoking history, Asian-Americans have a 66–100% greater incidence of lung cancer in comparison to non-Hispanic White Americans (44). This further extends to East Asian women in Asia and North America, who demonstrate a sizeable incidence of lung cancer though with a low prevalence of smoking. Specifically, nearly one-third of lung cancer cases in East Asian women occur in never-smokers and are believed to arise from complex genetic and environmental interactions (i.e., tobacco exposure in the workplace, environmental pollutants, and poor ventilation at home) (45). Unfortunately, limited inclusion of this demographic in screening trials implicitly suggests a missed opportunity to address factors that might influence disease development and health outcomes. The paradoxical associations seen in these communities infers an interplay of sociocultural and environmental factors, which require further clarification for lung cancer risk and specialized screening practices for specific minority groups.
Intersectionality of marginalized identities
The intersecting effects of race, SES and other social determinants on LCS uptake and adherence remain an area of interest for further study. Much of the existing literature and analyses of LCS patterns focus on the associations between independent social determinants. It is suspected that multiple marginalized identities are compounding factors that influence screening patterns. Further study of the associations between intersectionality is needed to target screening barriers and improve screening equity.
Addressing barriers to LCS and management
The health disparities in lung cancer care are in part manifestations of large screening trials with homogenous cohorts, which ultimately overlook more granular barriers faced by minority and marginalized groups. However, recent efforts towards health equity in LCS and management seem promising. The USPSTF 2021 recommendation for LCS implicitly suggests an awareness of existing disparities in lung cancer care. Access to care remains a major barrier for racial minorities and lower SES groups, though established federal infrastructure may offer insight on strategies to mitigate this disparity. Riviere et al. reported no difference in late-stage disease presentation or outcomes between African American men and non-Hispanic White men receiving prostate cancer care through the Veteran’s Health Administration (VHA), which contrasts national trends (46). This extends to lung cancer care as well, with a retrospective analysis of 18,466 patients in the Veteran Affairs Central Cancer Registry reporting an elimination of disparate treatment patterns between Black and White patients with early-stage lung cancer over a 10-year period (47). The VHA has clearly prioritized health equity and committed research on the social determinants of health, reducing and eliminating certain disparities encountered by the Black veteran community. While health inequities persist amongst other clinical situations and marginalized groups, the VHA experience suggests that in a centralized, equal-access healthcare system, racial disparities for cancer care can effectively be addressed. The generalizability of this infrastructure may be challenging across other populations that experience more clinical and social heterogeneity, though the VHA experience demonstrates the plausibility of reducing some racial disparities in regards to access to cancer care. A recent multicenter study of individuals undergoing LCS also reported no observed racial disparity in adherence to annual LCS at centralized programs, suggesting that a centralized LCS may improve screening equity (48). As health disparities research matures, shaping LCS practices in parallel with established federal models may address larger-scale health disparities seen in other marginalized groups.
Strategies toward health equity
Awareness of disparities in lung cancer care is a prerequisite for health equity, and the thoracic oncology community should be accountable for addressing inherent biases and inequalities in LCS, diagnosis, and treatment practices. Only with such conscious intention can strategies to overcome disparate screening practices prove effective.
Patient barriers to lung cancer care are multifactorial, but lack of awareness of screening availability and treatment potential for lung cancer seems to be a central issue, at least for marginalized groups. This has partly been addressed by the expansion of online and printable informational media by government efforts and professional societies. Providers and healthcare networks can also expand cancer care through community engagement. Inclusion of groups and services entrusted by minority groups in LCS promotion may also improve LCS participation. Faith-based outreach has been successful in delivering the message of screening through a patient-trusted, widely available source of information (49). Community-based clinics may also prove helpful, given that they have the infrastructure for socially competent messaging to engage individuals, with the potential to develop a protocol from clinic to screening to specialty care. It may also be prudent for healthcare groups to form partnerships with occupation-based health plans (i.e., law enforcement, firefighters, etc.) for high-risk professions to deliver appropriate screening.
Provider barriers, notably limited encounter time and awareness of screening risks, influence disparities in LCS as well. Physicians almost universally recommend LCS, though typically spend less than one minute discussing screening, with virtually no discussion of the potential harms of screening during patient visits (50). Shared decision-making, a process in which providers work with patients to tailor screening based on a patient’s individual risk of disease with the net effect of screening, has been shown to increase rates of favorable patient experiences and the knowledge base for LCS, especially in minority populations (51). Once referred to LCS by a provider, Black LCS-eligible patients have similar rates of screening participation to White LCS-eligible patients (52). While shared decision-making models may improve LCS practices, an additional provider barrier is the availability of sound follow-up infrastructure. A recent single-visit, multidisciplinary LCS model at an urban hospital network seems to improve patient satisfaction and with a positive effect on more equitable downstream management practices (53). With broadening of LCS criteria, individual providers need support of a multidisciplinary team to educate and guide patients through screening, adherence to annual LDCT, and follow-up care. Most importantly, LCS must integrate community and population-specific evidence when available as racial, ethnic and socioeconomic factors influence the differences in risk, detection and treatment of lung cancer.
Conclusions
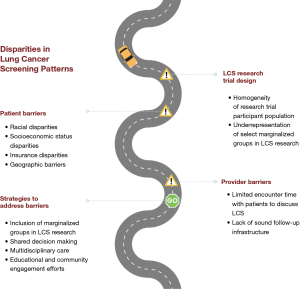
Results from multiple clinical trials have established the benefits of LCS, for high-risk individuals. Recent broadening of LCS screening criteria by the USPSTF and Centers for Medicare and Medicaid Services (CMS) to ages 50–80 years and 20 pack-years of smoking increases eligibility for this lifesaving technology, especially among Black and African Americans, though cannot individually reduce health inequities. Health disparities of LCS in the research, uptake, adherence and follow-up care persist. These disparities are complex and differ among different populations and individuals (Figure 1). Awareness of the foundational health disparities in cancer screening, as well as their extension into current LCS practice, is crucial for providers when considering a more patient-centered, multidisciplinary approach towards LCS and downstream interventions. Only then can screening utilization truly approach health equity and optimization. Additional population-specific research and patient-centered solutions for LCS implementation are needed to guide a sustainable, equitable future for LCS.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://ccts.amegroups.com/article/view/10.21037/ccts-22-4/rc
Peer Review File: Available at https://ccts.amegroups.com/article/view/10.21037/ccts-22-4/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts-22-4/coif). CPE is partially supported by Temple University/Fox Chase Cancer Center and Hunter College (TUFCCC/HC) Regional Comprehensive Cancer Health Disparity Partnership, Award Number U54 CA221704(5) (contact primary investigators: Grace X. Ma, PhD, and Olorunseun O. Ogunwobi, MD, PhD) from the National Cancer Institute of the National Institutes of Health (NCI/NIH) and is partially supported by Pfizer-American Cancer Society Health Disparity Award (contact primary investigators: Cherie P. Erkmen, MD and Grace X. Ma, PhD). CPE is a board member of Ride Hard Breathe Easy, the nonprofit organization for lung cancer patients advocacy. The content is the responsibility of the authors and does not represent the official views of the NCI/NIH, Pfizer, the American Cancer Society, or Ride Hard Breathe Easy. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med 2020;382:503-13. [Crossref] [PubMed]
- Xia W, Yu X, Mao Q, et al. Improvement of survival for non-small cell lung cancer over time. Onco Targets Ther 2017;10:4295-303. [Crossref] [PubMed]
- Huang KL, Wang SY, Lu WC, et al. Effects of low-dose computed tomography on lung cancer screening: a systematic review, meta-analysis, and trial sequential analysis. BMC Pulm Med 2019;19:126. [Crossref] [PubMed]
- Caverly TJ, Fagerlin A, Wiener RS, et al. Comparison of Observed Harms and Expected Mortality Benefit for Persons in the Veterans Health Affairs Lung Cancer Screening Demonstration Project. JAMA Intern Med 2018;178:426-8. [Crossref] [PubMed]
- Incze M, Redberg RF. Reducing Harms in Lung Cancer Screening-Bach to the Future. JAMA Intern Med 2018;178:326-7. [Crossref] [PubMed]
- Duma N, Vera Aguilera J, Paludo J, et al. Representation of Minorities and Women in Oncology Clinical Trials: Review of the Past 14 Years. J Oncol Pract 2018;14:e1-e10. [Crossref] [PubMed]
- Braveman P. What are health disparities and health equity? We need to be clear. Public Health Rep 2014;129:5-8. [Crossref] [PubMed]
- Aldrich MC, Mercaldo SF, Sandler KL, et al. Evaluation of USPSTF Lung Cancer Screening Guidelines Among African American Adult Smokers. JAMA Oncol 2019;5:1318-24. [Crossref] [PubMed]
- Adnan SM, Poulson M, Litle VR, et al. Challenges in the Methodology for Health Disparities Research in Thoracic Surgery. Thorac Surg Clin 2022;32:67-74. [Crossref] [PubMed]
- National Lung Screening Trial Research Team. Baseline characteristics of participants in the randomized national lung screening trial. J Natl Cancer Inst 2010;102:1771-9. Erratum in: J Natl Cancer Inst 2011 Oct 19;103(20):1560. [Crossref] [PubMed]
- Jemal A, Fedewa SA. Lung Cancer Screening With Low-Dose Computed Tomography in the United States-2010 to 2015. JAMA Oncol 2017;3:1278-81. [Crossref] [PubMed]
- Japuntich SJ, Krieger NH, Salvas AL, et al. Racial Disparities in Lung Cancer Screening: An Exploratory Investigation. J Natl Med Assoc 2018;110:424-7. [Crossref] [PubMed]
- Narayan AK, Chowdhry DN, Fintelmann FJ, et al. Racial and Ethnic Disparities in Lung Cancer Screening Eligibility. Radiology 2021;301:712-20. [Crossref] [PubMed]
- Prosper AE, Inoue K, Brown K, et al. Association of Inclusion of More Black Individuals in Lung Cancer Screening With Reduced Mortality. JAMA Netw Open 2021;4:e2119629. [Crossref] [PubMed]
- Tanner NT, Gebregziabher M, Hughes Halbert C, et al. Racial Differences in Outcomes within the National Lung Screening Trial. Implications for Widespread Implementation. Am J Respir Crit Care Med 2015;192:200-8. [Crossref] [PubMed]
- US Preventive Services Task Force. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021;325:962-70. [Crossref] [PubMed]
- Pu CY, Lusk CM, Neslund-Dudas C, et al. Comparison Between the 2021 USPSTF Lung Cancer Screening Criteria and Other Lung Cancer Screening Criteria for Racial Disparity in Eligibility. JAMA Oncol 2022;8:374-82. Erratum in: JAMA Oncol 2022 Dec 1;8(12):1856. [Crossref] [PubMed]
- Shin D, Fishman MDC, Ngo M, et al. The Impact of Social Determinants of Health on Lung Cancer Screening Utilization. J Am Coll Radiol 2022;19:122-30. [Crossref] [PubMed]
- Erkmen CP, Randhawa S, Patterson F, et al. Quantifying Benefits and Harms of Lung Cancer Screening in an Underserved Population: Results From a Prospective Study. Semin Thorac Cardiovasc Surg 2022;34:691-700. [Crossref] [PubMed]
- Pasquinelli MM, Kovitz KL, Koshy M, et al. Outcomes From a Minority-Based Lung Cancer Screening Program vs the National Lung Screening Trial. JAMA Oncol 2018;4:1291-3. [Crossref] [PubMed]
- Lake M, Shusted CS, Juon HS, et al. Black patients referred to a lung cancer screening program experience lower rates of screening and longer time to follow-up. BMC Cancer 2020;20:561. [Crossref] [PubMed]
- Erhunmwunsee L, Joshi MB, Conlon DH, et al. Neighborhood-level socioeconomic determinants impact outcomes in nonsmall cell lung cancer patients in the Southeastern United States. Cancer 2012;118:5117-23. [Crossref] [PubMed]
- Tseng TS, Gross T, Celestin MD, et al. Knowledge and attitudes towards low dose computed tomography lung cancer screening and smoking among African Americans-a mixed method study. Transl Cancer Res 2019;8:S431-42. [Crossref] [PubMed]
- Singh GK, Jemal A. Socioeconomic and Racial/Ethnic Disparities in Cancer Mortality, Incidence, and Survival in the United States, 1950-2014: Over Six Decades of Changing Patterns and Widening Inequalities. J Environ Public Health 2017;2017:2819372. [Crossref] [PubMed]
- Schaap MM, van Agt HM, Kunst AE. Identification of socioeconomic groups at increased risk for smoking in European countries: looking beyond educational level. Nicotine Tob Res 2008;10:359-69. [Crossref] [PubMed]
- Brown-Johnson CG, England LJ, Glantz SA, et al. Tobacco industry marketing to low socioeconomic status women in the U.S.A. Tob Control 2014;23:e139-46. [Crossref] [PubMed]
- Schütte S, Dietrich D, Montet X, et al. Participation in lung cancer screening programs: are there gender and social differences? A systematic review. Public Health Rev 2018;39:23. [Crossref] [PubMed]
- Castro S, Sosa E, Lozano V, et al. The impact of income and education on lung cancer screening utilization, eligibility, and outcomes: a narrative review of socioeconomic disparities in lung cancer screening. J Thorac Dis 2021;13:3745-57. [Crossref] [PubMed]
- Sosa E, D'Souza G, Akhtar A, et al. Racial and socioeconomic disparities in lung cancer screening in the United States: A systematic review. CA Cancer J Clin 2021;71:299-314. [Crossref] [PubMed]
- Steiling K, Loui T, Asokan S, et al. Age, Race, and Income Are Associated With Lower Screening Rates at a Safety Net Hospital. Ann Thorac Surg 2020;109:1544-50. [Crossref] [PubMed]
- Brouwers MC, De Vito C, Bahirathan L, et al. What implementation interventions increase cancer screening rates? a systematic review. Implement Sci 2011;6:111. [Crossref] [PubMed]
- Kale MS, Wisnivesky J, Taioli E, et al. The Landscape of US Lung Cancer Screening Services. Chest 2019;155:900-7. [Crossref] [PubMed]
- Odahowski CL, Zahnd WE, Eberth JM. Challenges and Opportunities for Lung Cancer Screening in Rural America. J Am Coll Radiol 2019;16:590-5. [Crossref] [PubMed]
- Rohatgi KW, Marx CM, Lewis-Thames MW, et al. Urban-Rural Disparities in Access to Low-Dose Computed Tomography Lung Cancer Screening in Missouri and Illinois. Prev Chronic Dis 2020;17:E140. [Crossref] [PubMed]
- Bradley CJ, Given CW, Roberts C. Disparities in cancer diagnosis and survival. Cancer 2001;91:178-88. [Crossref] [PubMed]
- Bradley CJ, Dahman B, Given CW. Treatment and survival differences in older Medicare patients with lung cancer as compared with those who are dually eligible for Medicare and Medicaid. J Clin Oncol 2008;26:5067-73. [Crossref] [PubMed]
- Tammemägi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med 2013;368:728-36. [Crossref] [PubMed]
- Fiscella K, Winters P, Farah S, et al. Do Lung Cancer Eligibility Criteria Align with Risk among Blacks and Hispanics? PLoS One 2015;10:e0143789. [Crossref] [PubMed]
- Holmes JA, Chen RC. Racial Disparities in Time From Diagnosis to Treatment for Stage I Non-Small Cell Lung Cancer. JNCI Cancer Spectr 2018;2:pky007. [Crossref] [PubMed]
- Yanez B, McGinty HL, Buitrago D, et al. Cancer Outcomes in Hispanics/Latinos in the United States: An Integrative Review and Conceptual Model of Determinants of Health. J Lat Psychol 2016;4:114-29. [Crossref] [PubMed]
- Price SN, Flores M, Hamann HA, et al. Ethnic Differences in Survival Among Lung Cancer Patients: A Systematic Review. JNCI Cancer Spectr 2021;5:pkab062. [Crossref] [PubMed]
- DeRouen MC, Canchola AJ, Thompson CA, et al. Incidence of Lung Cancer Among Never-Smoking Asian American, Native Hawaiian, and Pacific Islander Females. J Natl Cancer Inst 2022;114:78-86. [Crossref] [PubMed]
- Zhou F, Zhou C. Lung cancer in never smokers-the East Asian experience. Transl Lung Cancer Res 2018;7:450-63. [Crossref] [PubMed]
- Riviere P, Luterstein E, Kumar A, et al. Survival of African American and non-Hispanic white men with prostate cancer in an equal-access health care system. Cancer 2020;126:1683-90. [Crossref] [PubMed]
- Williams CD, Salama JK, Moghanaki D, et al. Impact of Race on Treatment and Survival among U.S. Veterans with Early-Stage Lung Cancer. J Thorac Oncol 2016;11:1672-81. [Crossref] [PubMed]
- Kim RY, Rendle KA, Mitra N, et al. Racial Disparities in Adherence to Annual Lung Cancer Screening and Recommended Follow-Up Care: A Multicenter Cohort Study. Ann Am Thorac Soc 2022;19:1561-9. [Crossref] [PubMed]
- Hou SI, Cao X. A Systematic Review of Promising Strategies of Faith-Based Cancer Education and Lifestyle Interventions Among Racial/Ethnic Minority Groups. J Cancer Educ 2018;33:1161-75. [Crossref] [PubMed]
- Brenner AT, Malo TL, Margolis M, et al. Evaluating Shared Decision Making for Lung Cancer Screening. JAMA Intern Med 2018;178:1311-6. [Crossref] [PubMed]
- Sferra SR, Cheng JS, Boynton Z, et al. Aiding shared decision making in lung cancer screening: two decision tools. J Public Health (Oxf) 2021;43:673-80. [Crossref] [PubMed]
- Kunitomo Y, Bade B, Gunderson CG, et al. Evidence of Racial Disparities in the Lung Cancer Screening Process: a Systematic Review and Meta-Analysis. J Gen Intern Med 2022;37:3731-8. [Crossref] [PubMed]
- Erkmen CP, Moore RF, Belden C, et al. Overcoming Barriers to Lung Cancer Screening by Implementing a Single-Visit Patient Experience. Int J Cancer Oncol 2017; [PubMed]
Cite this article as: Adnan SM, Chin K, Ma GX, Erkmen CP. A narrative review of the social determinants of lung cancer screening: knowledge gaps and controversies. Curr Chall Thorac Surg 2023;5:41.