Robotic-assisted left lower lobectomy
Highlight box
Surgical highlights
• This is an original manuscript describing the technical details of performing a robotic assisted left lower lobectomy using a four-arm technique on the Davinci Xi system.
What is conventional and what is novel/modified?
• Here we describe the surgical technique of a robotic assisted left lower lobectomy, divided in key basic steps. This is not the only sequence for performing this surgery, but it highlights the key steps and basic skills needed to perform the surgery in a safe and efficient matter.
What is the implication, and what should change now?
• Surgeons looking to start a robotic approach to lobectomy will find some recommendations and examples on how to perform the operation in several steps,
Introduction
Robotic surgery provides superior visualization of the surgical field with a 3D camera and 10× magnification. In addition, articulating instruments and a stable platform allows control of the dissection with enhanced precision and control. Despite controversies regarding the benefit of a robotic approach over well-established video-assisted thoracoscopic surgery (VATS) techniques, the adoption of robotic-assisted thoracoscopic surgery (RATS) for lung cancer continues to increase, with a consequent decrease in the use of open thoracotomy for lobectomy (1). In this chapter, we focus on the technical aspects of a robotic assisted left lower lobectomy for lung cancer. The left lower lobe is technically simpler to perform given the more consistent anatomy and infrequent anatomic variations. However, some cases can be more challenging due to tumor size, adenopathy or anatomic difficulties. Having a consistent approach to RATS lobectomy facilitates the speed and safety of the operation and allows for a more efficient procedure. Of course, one must be able to change course and customize the approach for each patient, but in general, simplifying the operation into key steps can help the surgeon master the operation and increase the efficiency and safety of the surgery.
Surgical technique
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and the accompanying images and video. A copy of the written consent is available for review by the editorial office of this journal.
Patient positioning and setup
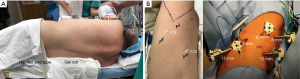
Room setup and the conduct of the case is similar to any other pulmonary robotic procedure. We aim to standardize as many aspects of the case as possible in order to decrease inter-case variability and enhance efficiency. The patient is placed right lateral decubitus position with the table flexed at the hip. The arms are positioned in a neutral position with a pillow between them or in a resting arm board (Figure 1A). Lung isolation is done with a left sided double lumen tube as our preference, but other techniques such as bronchial blockers are also possible.
Port placement and docking
Port placement is critical for any robotic lobectomy. Optimal spacing between the ports is essential to minimize collisions and chest wall trauma. We aim for 8 cm of separation between all ports. Also, the most posterior trocar is placed at least 8 cm away from the spinous process. Keep in mind that is certain patients with small body habitus or thin patients a tighter port placement strategy with less separation may be needed, with the potential for collitions, therefore, planning the port placement before the initial incision is recommended. It is important to be at the adequate intercostal space to be able to optimize visualization and stapling angles. We find that the 7th or 8th intercostal space (ICS) is the most reliable space for pulmonary resections, and we choose the widest space of the two, as long as the ports are one or two intercostal spaces below the major fissure (Figure 1B). Several considerations are to be taken into account when selecting port locations: Stapling angles, camera view, assistant location and specimen extraction. We prefer to have a posterior robotic stapling port which is reusable and allows posterior stapling with robotic platform when needed, but for lower lobectomy a standard assistant non-robotic trocar is also feasible. Docking of the robotic system starts by establishing a clear path to drive the robot into the desired location. We try to have a consistent robot cart entry path for every lung resection case. For an Si robotic system, we find it easiest to drive the robot over the patient’s head. For the Xi system given that the boom can rotate, we bring the cart over the patient’s shoulder at 30-degree angle off the head, to allow for more space for the anesthesia team. We point to the camera port for the staff and the laser guidance cross mark can be aligned with the camera port easily.
Sequence of the operation
A full video of a robotic left lower lobectomy is available for review (Video 1). A robotic left lower lobectomy starts with a lymphadenectomy. This allows a complete nodal dissection while the field is bloodless and opens the spaces to facilitate structure isolation and stapler passage. Depending on the patient’s anatomy, the fissure may be dissected next, otherwise other methods to avoid the fissures and air leaks are described below. The instrument choice is a matter of personal preference. We find that a retracting arm is best with the tip-up grasper (arm 4), a dissecting tool is preferred in the right hand with a curved bipolar dissector or similar fine bipolar tool (arm3), the camera is in arm 2, and a grasping tool such as a Cadiere forceps is placed in the most anterior (arm 1).
The following are steps for a standard left lower lobectomy:
- Step 1. Division of inferior pulmonary ligament and inferior nodes (L8, L9).
Standard for every robotic lobectomy, we start by superior lung retraction releasing the inferior ligament. This allows further drop of the diaphragm and great exposure of the infrahilar lymph nodes. This also leads to dissection of the inferior pulmonary vein (IPV) and surrounding lymph nodes. - Step 2. Dissection of posterior hilum, pulmonary artery and posterior nodes (L7, L10, L11).
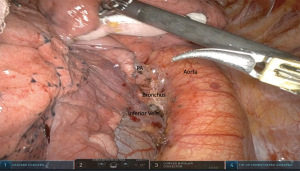
Next, anterior retraction of the lung by pushing the posterior arm 4 instrument exposes the subcarinal space for the level 7 nodal dissection. Next, dissection of the posterior level 10L lymph nodes will create an avascular plane directly over the pulmonary artery (Figure 2). This plane is followed anteriorly towards the fissure to create a posterior tunnel. Then the same plane is created posterior to the artery to separate it from the lower lobe bronchus. - Step 3. Dissection of anterior hilum between the pulmonary veins and anterior nodes (10L).
The lung is than allowed to fall back to normal and retracted posteriorly, in order to dissect the anterior aspect of the veins. This ensure preserving an intact superior pulmonary vein and rule out a common venous trunk. Further dissection clears the anterior level 10L and 11L nodes exposing again the pulmonary artery and the lower lobe bronchus. - Step 4. Dissection of interlobar pulmonary artery, hilar nodes (11L, 12L), fissure completion.
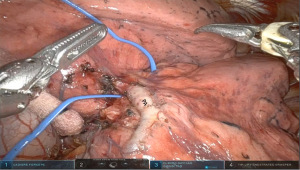
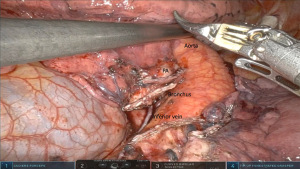
After the hilar dissection the planes are now well dissected and the interlobar pulmonary artery is often easily identified. This then facilitates completing the fissure anteriorly and posteriorly. Care must be taken to avoid injury to the lingular artery branch while completing the anterior fissure with the stapler, which is driven from the anterior arm 1. In cases of incomplete fissures, one can avoid parenchymal air leaks by developing a tunnel anteriorly in between the pulmonary veins until reaching the interlobar level 11L nodes. Removing these can identify the lingular branch of the artery and the lower lobe basilar branch below. Starting the staling of the fissure superior to these vessels is then safe. Alternatively, an inferior to superior approach is performed. The inferior vein is divided, followed by dissection of the lower lobe bronchus crating a separating from the basilar and superior segment arteries. One can then encircle and divide the bronchus taking care not to injure the artery above. After this it is clear to isolate the fissure and the artery (Figure 3). - Step 5. Isolation and division of pulmonary arteries lower lobe (A6–10).
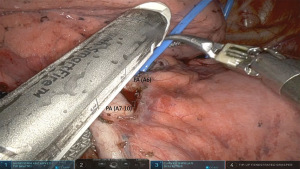
With the fissure complete, it is much easier to have a complete view of the lower lobe pulmonary artery. Preferably a large window is created between the artery and the lower lobe bronchus where the entire lower lobe basilar and superior segment branches are encircled with a vessel loop and divided with a vascular stapler from the anterior arm 1 (Figure 4). Otherwise, if the visualization is incomplete or in case of a high take off of the superior segment branch (A6), the vessels can be divided individually. - Step 6. Isolation and division of IPV.
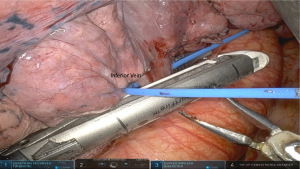
Next, superior and anterior retraction exposes the IPV. This is encircled and divided with another vascular stapler from arm 1 (Figure 5). - Step 7. Isolation and division of lower lobe bronchus.
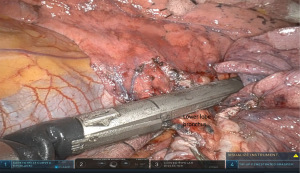
At this point, further dissection of the level 11L and 12 L lymph nodes exposes well the lower lobe bronchus. I find it helpful to lift he lower lobe away from the pericardium and descending aorta, with easy passage of a green or black load stapler across the lower lobe bronchus from arm 1 (Figure 6). In cases where there is concern about margins or airway invasion, the airway can be transected and oversewn with absorbable suture in running or interrupted fashion - Step 8. Dissection of superior mediastinum AP window (5L, 6L).
At this point with the lobectomy specimen aside, the nodal dissection is completed in the superior mediastinum and Aorta pulmonary window. - Step 9. Specimen extraction and undocking.
The specimen can be placed in an endo catch bag and retrieved though an extended arm 1 port site. The final hilar dissection and structures can be visualized and inspected (Figure 7).
Comments
Robotic assisted lobectomy is feasible and safe (2). The left lower lobectomy approached by RATS is feasible and it is an ideal operation to master during the robotic learning curve (3). However, the benefits of robotic surgery over well-established VATS technique are controversial in terms of length of stay, blood loss and recovery (4). Even with these findings, thoracic surgeons are slowly adapting to (RATS) due to improved dexterity, visualization and the ability to progress into more complex resections (1). Left lower lobectomy presents an opportunity for beginning robotic thoracic surgeons to master robotic lung resection, given lesser degree of anatomic variation, and dissection. Robotic lobectomy techniques are now well established and are becoming an important tool for thoracic surgeon looking to expand their armamentarium for pulmonary resection. The ability to do a completely portal technique, with CO2 insufflation and articulating instrument allows for meticulous dissection and optimal visualization which can make a difference in cases of complex resections (5). The big question is whether RATS is superior to VATS as an approach for minimally invasive lung resection in particular, for a straight forward left lower lobectomy. Despite the lack of randomized clinical trials, it has been suggested that some of the advantages of the robotic platform include the superior three-dimensional perspective, magnification, and instrument articulation (6). These benefits can make a difference in the successful completion of a complex resection, or in avoiding a conversion to open thoracotomy due to lack of progress during the dissection. In a recent meta-analysis, Ma and colleagues performed a comprehensive literature review of reported studies totalling 11,247 patients comparing VATS and RATS for lung cancer resection (7). The study reported that RATS had a lower blood loss, conversion rate, length of stay and complications, and more harvested lymph nodes compared to VATS, but at a higher cost of care (7). However, in a national database study in the United States, Alwatari and colleagues found an increasing adoption of RATS utilization over time compared to VATS, but with no significant difference in length of stay or complications, but higher costs were associated with the robotic approach (8). Over time, large cohort propensity matched studies or randomized trials will hopefully answer the question of benefit and cost effectiveness of a robotic approach. Until then, it has become an essential tool for lung cancer treatment in our practice.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Richard Lazzaro) for the series “Robotic Anatomic Pulmonary Resection” published in Current Challenges in Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts-20-158/coif). The series “Robotic Anatomic Pulmonary Resection” was commissioned by the editorial office without any funding or sponsorship. LJH reports personal fees from Intuitive Surgical Inc, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and the accompanying images and video. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Oh DS, Reddy RM, Gorrepati ML, et al. Robotic-Assisted, Video-Assisted Thoracoscopic and Open Lobectomy: Propensity-Matched Analysis of Recent Premier Data. Ann Thorac Surg 2017;104:1733-40. [Crossref] [PubMed]
- Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9. [Crossref] [PubMed]
- Zirafa CC, Davini F, Romano G, et al. Robotic Lobectomy: Left Lower Lobectomy by Surgery. Oper Tech Thorac Cardiovasc Surg 2017;22:43-57. [Crossref]
- Louie BE, Wilson JL, Kim S, et al. Comparison of Video-Assisted Thoracoscopic Surgery and Robotic Approaches for Clinical Stage I and Stage II Non-Small Cell Lung Cancer Using The Society of Thoracic Surgeons Database. Ann Thorac Surg 2016;102:917-24. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Mazzei M, Abbas AE. Why comprehensive adoption of robotic assisted thoracic surgery is ideal for both simple and complex lung resections. J Thorac Dis 2020;12:70-81. [Crossref] [PubMed]
- Ma J, Li X, Zhao S, et al. Robot-assisted thoracic surgery versus video-assisted thoracic surgery for lung lobectomy or segmentectomy in patients with non-small cell lung cancer: a meta-analysis. BMC Cancer 2021;21:498. [Crossref] [PubMed]
- Alwatari Y, Khoraki J, Wolfe LG, et al. Trends of utilization and perioperative outcomes of robotic and video-assisted thoracoscopic surgery in patients with lung cancer undergoing minimally invasive resection in the United States. JTCVS Open 2022;12:385-398. [Crossref] [PubMed]
Cite this article as: Herrera LJ, Ocean G. Robotic-assisted left lower lobectomy. Curr Chall Thorac Surg 2023;5:44.