Intrathoracic muscle flap transposition: an original surgical technique
Highlight box
Surgical highlights
• Once the empyema cavity is adequately debrided and the volume of the residual cavity reduced, the intrathoracic flap must be attached all around the fistula and properly anchored.
What is conventional and what is novel/modified?
• Conventional thoraco-myoplasty requires demolitive surgery and long hospitalization.
• Our computed tomography-guided wire allowed the muscle flap to be fixed in the most suitable site without a thoracotomy. The introduction of the two Vicryl traction sutures was essential for anchoring the flap, allowing it to remain in place during coughing and air leakage.
What is the implication, and what should change now?
• Open window thoracostomy and negative-pressure wound therapy are still effective procedures in reduce and sterilize empyema cavity complicated by bronchopleural fistula. Intrathoracic muscle flap transposition often is the only way to finally seal the fistula. Our technique proved to be effective without reopening the chest even though further experiences are needed to validate it.
Introduction
Background
Bronchopleural fistula (BPF) is defined as a communication between a main stem, lobar, or sublobar bronchus with the pleural space. The incidence of BPF is about to 1% for lobectomy and it increases up to 20% after pneumonectomy, with a mortality ranged up to 50%. BPF should always be suspected if a patient shows persistent air leak and it can be associated and complicated by pleural empyema. Patients with BPF and pleural empyema after pulmonary resection can be treated by the open window thoracostomy (OWT) in order to control infection and promote fistula closure (1).
Rationale
The use of negative-pressure wound therapy (NPWT) can improve the effectiveness of OWT (2) to clean and to reduce the volume of the infected cavity, favoring the closure of the fistula (3). Often, a redo thoracotomy is usually needed. If the treatment does not lead to fistula closure, an effective therapeutic solution can be filling the residual pleural cavity with well-vascularized tissue, such as intrathoracic muscle flaps (4) and/or omentum (5).
Objective
For a successful surgical outcome, the correct fixation of the intrathoracic flap in close proximity to the fistula is one of the essential technical prerequisites.
This article describes a new technique for fixation of a muscle flap, transposed into the chest, through a long-standing OWT, without a redo thoracotomy. We present this article in accordance with the SUPER reporting checklist (available at https://ccts.amegroups.com/article/view/10.21037/ccts-23-6/rc).
Preoperative preparations and requirements
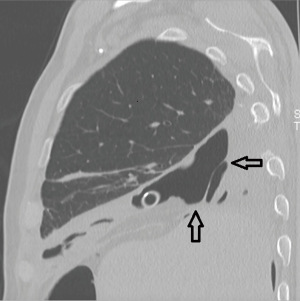
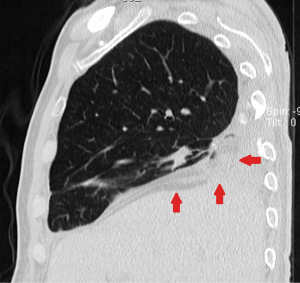
A 70-year-old male patient was referred to our Institution in April 2022 for the treatment of a lung adenocarcinoma of the right lower lobe (cT2aN0M0). Pulmonary and cardiac function tests were in the normal range. About 50 years earlier, the patient underwent right thoracotomy and subtotal pleurectomy for recurrent pneumothoraxes. Surgical risks and possible alternatives were clearly explained to the patient, who accepted for surgical treatment. The right lower lobectomy was technically demanding due to complete pleural symphysis and calcified hilar lymph nodes. In order to avoid residual pleural cavity, 800 mL pneumoperitoneum was instituted intraoperatively. On the 3rd postoperative day, surgical re-exploration was carried out for mild but persistent bleeding. The source of hemorrhage was identified at the level of the denuded intrathoracic fascia and hemostasis was temporarily achieved by electrocautery and hemostatic sponges. On the 6th postoperative day, an angiography was performed because the hemostasis was still unsatisfactory: all the right intercostal arteries were evidently hypertrophied and were embolized (from the 3rd through the 9th) with immediate bleeding control. The postoperative course was then uneventful and the patient was discharged, despite a persistent basal residual pleural cavity. About 45 days later the patient experienced cough and septic fever. Bronchoscopy revealed a small BPF, opening into the enlarged residual basal pleural cavity, which proved to be contaminated by Enterococcus faecium infection. The fistula was ascribed to three determinants: length of the bronchial stump, suture devascularization induced by the intercostal arteries embolization and persistent pleural cavity (Figure 1).
Due to the poor general condition of the patient, direct closure of the fistula was deemed not indicated. An OWT was created by removing the 5th and 6th ribs in the mid-axillary line allowing for complete debridement of the pleural space and removal of purulent material. NPWT at −30 mmHg was then instituted and air leaks were reduced by bronchoscopic instillation of submucosal human fibrin glue. Targeted antibiotic therapy and periodic medications allowed local and systemic infection to be controlled in about 5 weeks. Because of fistula enlargement, bronchial filling by endobronchial Watanabe spigot (6) and endobronchial valve were positioned in order to achieve the air leak control (7).
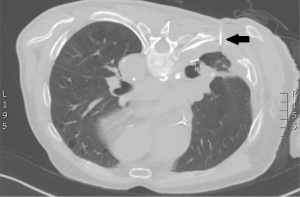
After 6 months, the volume of the residual pleural cavity (Figure 2) decreased but the BPF underwent progressive enlargement, affecting almost the entire bronchial stump.
In order to seal the fistula and to fill the residual pleural cavity we decided to use the right rectus abdominis muscle (RAM) flap, since the latissimus dorsi muscle and the omentum were not available for previous surgery. The RAM flap was chosen after careful preoperative measurement of its theoretical length and volume. In the surgical planning the main issue was how to attach the intrathoracic muscle flap in the right position within the old, rigid and narrow thoracostomy cavity, without reopening the chest. The original technical solution adopted is presented below.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and the accompanying images and video. A copy of the written consent is available for review by the editorial office of this journal.
Step-by-step description
The ideal site for the muscle fap fixation was identified by preoperative computed tomography (CT) scan and bronchoscopy at the bottom of the thoracostomy cavity, close to the dehiscent bronchial stump. At this level, a hookwire was posteriorly placed under CT guidance, immediately before surgery (Figure 3). The patient was intubated with a single lumen endotracheal tube and placed in the supine position: the right RAM flap based on the superior epigastric vessels was set up and placed in a subcutaneous pocket at the level of the right costal arch.
With the patient in left lateral decubitus, a 3 mm 30-degree telescope was inserted into the thoracostomy cavity and two trans-parietal 14-gauge BD Venflon™ Pro Safety needles were introduced into the cavity next and parallel to the metal hookwire, one at the same level, the second at the level of the lower intercostal space (Video 1). Two transthoracic 2-0 Vicryl sutures were driven into the cavity through the Venflon needles and retrieved from inside, under thoracoscopic guidance. One end of the Vicryl sutures was left outside the chest and the other end was sutured to the RAM flap fascia with a free needle. The external ends of the Vicryl sutures were then gently pulled from outside so that the muscle flap, anchored to them, was transposed into the cavity and driven at the desired site, close to the fistula. Once the correct placement of the muscle flap has been verified, the external ends of the Vicryl sutures were knotted each other around the contiguous rib element.
Postoperative considerations and tasks
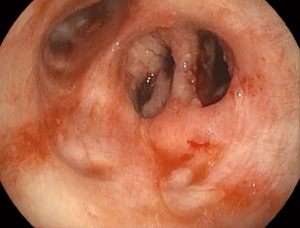
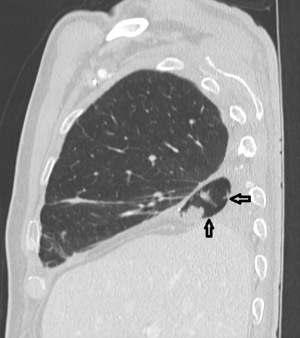
The duration of the whole procedure was about 180 minutes. The patient was extubated immediately after surgery. The postoperative course was uneventful. On day 13, chest CT scan showed the intrathoracic muscle flap filling the basal residual space, which was almost completely obliterated (Figure 4). The day after, video bronchoscopy demonstrated the sealing of the bronchial stump by the contiguous muscle fap (Figure 5). The discharge took place on day 17. After 12 months the patient is alive and in excellent condition.
Tips and pearls
Once the empyema cavity has been adequately debrided and the volume of the residual cavity reduced, a CT-guided wire allows the muscle flap to be fixed in the most suitable site without the need for a thoracotomy. The intrathoracic is then attached in close proximity to the fistula and properly anchored in the cavity by two transthoracic 2-0 Vicryl sutures, previously driven into the cavity by two Venflon needles.
Discussion
OWT is an effective method to debride the empyema cavity secondary to BPF, thus controlling local and systemic infection (8). NPWT, after repeated dressings, promotes the cavity sterilization and granulation tissue growth, and reduces the volume of the residual space, possibly sealing the fistula. Temporary plugging of large fistulas is required in order to avoid excessive air leakage (9). If the procedure does not lead to the fistula closure, the use of intrathoracic tissue flaps can allow definitive healing by closing the fistula and obliterating the residual space with well-vascularized tissue.
The key to success is summed up in three basic steps: (I) the empyema cavity must be adequately debrided; (II) the volume of the residual cavity should be reduced as possible and must be proportionate to the volume of the intrathoracic flap to be used; and (III) the flap must be attached in close proximity to the fistula, possibly around it.
The first two steps can be reached by OWT and NPWT after plugging of the fistula. The third point usually requires a redo thoracotomy because a flap not adequately fixed around the fistula is a candidate for failure. Multiple surgeries, months of dressings, prolonged antibiotic therapy, and long-standing sepsis make the redo thoracotomy not well tolerated by the patient. Furthermore, opening the chest again would have re-enlarged the cavity, which was reduced by the previous treatments.
We developed this technique, starting from the experience with the use of CT-guided wires, that can be placed very close to the dehiscent bronchial stump, after a right lower lobectomy. The metal wire allows the intrathoracic muscle flap to be anchored with transthoracic stitches in the most suitable site without the need for a thoracotomy. Another essential requirement is the volume of the flap which must be greater/equal to the volume of the cavity. Indeed, this method does not allow the flap to be fixed around the fistula, which may not be sealed if the cavity is not completely obliterated.
We decided to use the ipsilateral RAM flap because both the latissimus dorsi and the omentum were not available due to previous surgery. The intrathoracic myoplasty was performed after several months of NPWT, only when the residual cavity volume has been deemed to be smaller than the presumed volume provided by the RAM flap. The posterior trans-parietal introduction of the two Vicryl traction sutures was essential for anchoring the flap at the bottom of the space, allowing it to remain in place during coughing and air leakage, finally sealing the fistula.
Conclusions
To treat BPF and residual pleural cavity, the use of a CT-guided hookwire to perfectly locate the bronchopleural stump and the use of the Venflon needle to deliver suture stitches to fix the flap through a narrow OWT, are the two key points of our technique. To date, no intrathoracic muscle flap transposition techniques that do not involve reopening the chest are described in the literature. Our technique was effective but further experiences are needed to validate it.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://ccts.amegroups.com/article/view/10.21037/ccts-23-6/rc
Peer Review File: Available at https://ccts.amegroups.com/article/view/10.21037/ccts-23-6/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts-23-6/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and the accompanying images and video. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wotton R, Garner M, Salem A, et al. Is open window thoracostomy the only method to control infection in patients with an empyema following pulmonary resection for primary lung cancer? Interact Cardiovasc Thorac Surg 2021;32:928-32. [Crossref] [PubMed]
- Nishii K, Nakajima T, Yamamoto T, et al. Management of thoracic empyema with broncho-pulmonary fistula in combination with negative-pressure wound therapy. Gen Thorac Cardiovasc Surg 2021;69:843-9. [Crossref] [PubMed]
- Iwasaki M, Shimomura M, Ii T. Negative-pressure wound therapy in combination with bronchial occlusion to treat bronchopleural fistula: a case report. Surg Case Rep 2021;7:61. [Crossref] [PubMed]
- Asaad M, Van Handel A, Akhavan AA, et al. Muscle Flap Transposition for the Management of Intrathoracic Fistulas. Plast Reconstr Surg 2020;145:829e-38e. [Crossref] [PubMed]
- Puma F, Fedeli C, Ottavi P, et al. Laparoscopic omental flap for the treatment of major sternal wound infection after cardiac surgery. J Thorac Cardiovasc Surg 2003;126:1998-2002. [Crossref] [PubMed]
- Miyahara E, Ueda D, Kawasaki Y, et al. Thoracoscopic surgery with Endobronchial Watanabe Spigot (EWS) is an effective treatment against empyema with fistula. Jpn J Chest Surg 2018;32:830-6. [Crossref]
- Schweigert M, Kraus D, Ficker JH, et al. Closure of persisting air leaks in patients with severe pleural empyema--use of endoscopic one-way endobronchial valve. Eur J Cardiothorac Surg 2011;39:401-3. [Crossref] [PubMed]
- Regnard JF, Alifano M, Puyo P, et al. Open window thoracostomy followed by intrathoracic flap transposition in the treatment of empyema complicating pulmonary resection. J Thorac Cardiovasc Surg 2000;120:270-5. [Crossref] [PubMed]
- Dugan KC, Laxmanan B, Murgu S, et al. Management of Persistent Air Leaks. Chest 2017;152:417-23. [Crossref] [PubMed]
Cite this article as: Coviello E, Napolitano AG, Pourmolkara D, Ceccarelli S, Colafigli C, Puma F. Intrathoracic muscle flap transposition: an original surgical technique. Curr Chall Thorac Surg 2023;5:50.