Vein-first strategy for thoracoscopic lung segmentectomy under use of three-dimensional reconstruction of computed tomography
Highlight box
Key findings
• We introduce the vein-first approach to begin lung segmentectomy procedure.
What is conventional and what is novel/modified?
• The conventional method of segmentectomy is to manage the artery and bronchus first, and then manage the veins dividing the intersegmental planes.
• We introduced managing the veins first to begin lung segmentectomy.
What is the implication, and what should change now?
• The vein-first approach strategy under three-dimensional reconstruction of multidetector computed tomography assessment can make the operation straightforward and prevent convergence in some situations of segmentectomy.
Introduction
Lung segmentectomy is in great demand as small-sized or minimally invasive lung cancers have been increasing and the recent findings from the JCOG0802 and CALGB140503 randomized control trials have showed favorable results of segmentectomy (1,2). Usually, the textbook of segmentectomy describes to start the operation by isolating and dividing the artery and bronchus at first, and the dissection of veins is to be done later (3). However, we found managing the vein-first sometimes merits the operation from the technical point of view during the development of thoracoscopic segmentectomy under planning with preoperative three-dimensional reconstruction of multidetector computed tomography (3D-CT) use (4,5). To perform a precise anatomical segmentectomy, if we treat the artery and bronchus first, we usually need to dissect the intersegmental plane later, seeking venous branches. If we expose the intersegmental veins first and divide the intra segmental veins, the bronchus and arteries will spontaneously be peeled off and become exposed and apparent easily. We realized this would eliminate unnecessary maneuvers when we precisely grasp the anatomy by 3D-CT. It led us to apply the technique to various segments. We present this article in accordance with the SUPER reporting checklist (available at https://ccts.amegroups.com/article/view/10.21037/ccts-23-9/rc).
Preoperative preparations and requirements
We recorded multidetector computed tomography (MDCT) images by injecting an iodinated contrast medium and saved the digital imaging and communications in medicine (DICOM) data on a server. We mainly used workstations or a client viewer for image analyses and determined pulmonary arteriovenous structure using a three-dimensional (3D) reconstruction. The surgeon processed the 3D images and used them to identify (I) the segmental arterial branches; (II) the intersegmental veins that were to be preserved; and (III) the venous branches in the affected segment (intrasegmental veins) that were to be divided. We also used them in the operating room to create and manipulate similar images magnified, de-magnified, or rotated during surgery with real-time conditions (4,5). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Data collection and analyses were approved by the ethics committee of the Faculty of Medicine, Yamagata University (IRB No. 2023-259). Written informed consent was obtained from the patient for publication of this article and the accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Step-by-step description
The thoracoscopic segmentectomy procedure was performed under general anesthesia with bilateral lung ventilation. The patient was placed in the lateral decubitus position. The surgeon usually stood on the patient’s ventral side and the assistants on the dorsal side. One 20-mm flexible utility port was placed in the fourth or fifth intercostal space of the anterior axillary line, and three 5-mm ports were placed in a multiport approach. A single 3.0–4.0 cm skin incision was made in the third, fourth, fifth, or sixth intercostal space of the anterior axillary line and the surgical wound was covered with a wound retractor in a single port approach. An assistant surgeon controlled the 5-mm, 30-degree endoscope.
We first dissect hilar parenchyma along the inter-segmental veins if they are present under the analysis of 3D-CT in addition to two-dimensional thin-sliced images. After dissection of the pulmonary venous branches with the division of intrasegmental venous confluences, the pulmonary arterial or bronchial branches become more straightforward, then we can manage the artery and bronchus. In further dissection of the hilar pulmonary parenchyma along inter segmental veins, we divide the intersegmental plane using staplers by either the inflation-deflation lines (6-8) or indocyanine green (ICG) fluorescent method (9,10). We describe the detailed manipulation of this method in typical and some atypical segmentectomy procedures. We describe below the operative procedures in each segmentectomy.
Right S1
We expose the V1 from the upper pulmonary vein (PV) and isolate the V1a and V1b at the mediastinal side of the upper lobe. We dissect the lung parenchyma along the intersegmental V1b which runs between S1 and S3 about 2 to 3 cm and divide the intrasegmental V1a which drains the S1 and runs between S1a (posterior S1) and S1b (anterior S1) subsegment. This procedure makes the isolation of A1 straightforward. The central vein which drains S1 is isolated after division of B1.
We usually find the confluence of the proximal intra-segmental venous branches and divide those using energy devices and the dissection of the hilar lung parenchyma between these veins releases the hilum side. The peripheral lung parenchyma is divided using staplers using either inflation-deflation line or ICG fluorescence, thus segmentectomy is completed. The procedure for the peripheral lungs is usually the same as above in other segments; therefore, we will describe the following with respect to the pulmonary hilar structures.
Right S2
We dissect the V2, isolate the central vein and V2c, and then incise the proximal lung parenchyma along the V2c. After the division of ascending A2, further dissection of the central vein, preferably inside the vascular sheath to the cranial side, leads to the exposure of B2 in a spontaneous sequence. V2b is divided; however, whether dividing or preserving V2a depends on the nature of the tumor and the location.
Right S3
Precise assessment of venous branches using 3D-CT and isolation of V1b and V2c after division of V3b and V3a is the key to accurate segmentectomy. We dissect the lung parenchyma along V1b and V2c and manage the A3 and B3 (Video 1). The broad surface of the central vein becomes visible, and its thorough observation of the V2 central vein will provide evidence of an accurate S3 resection (Figure 1). Surgeons should consider that the resected segment may be only S3b when the central vein is not observed on the segmentectomy plane.
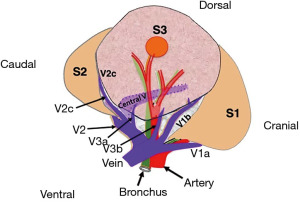
We prefer to preserve V2 central vein in S2 or S3 segmentectomy as it is the main drainage trunk of the remaining segments. We will choose lobectomy when we consider the need for combined resection of the central vein.
Right S4 and S5
In segmentectomy of the middle lobe, the assessment of venous running by CT is essential. If there is an intersegmental vein, the hilar parenchymal incision along the vein will smoothly reveal the bronchus, and after the bronchial division the pulmonary artery should be treated. If the vein is running along the bronchus, we divide the vein, then manage the bronchus and artery. This method is particularly suitable when there is an incomplete fissure between the upper and middle lobes.
Right S6
Right S6 segmentectomy is usually preceded by management of A6 and B6, and is not subject to the vein-first approach. However, we chose the vein-first approach when there is an incomplete fissure. We divide V6 or V6a initially, then divide the B6 and A6 from the posterior side, and open the intersegmental plane along the V6b,c, and finally divide the interlobar fissure.
Right S7 and S8
Right S7 or S8 segmentectomy is not the usual candidate for the vein-first strategy. We use this method when the interlobar fissure is incomplete, or the right B7 is posterior to the common basal vein.
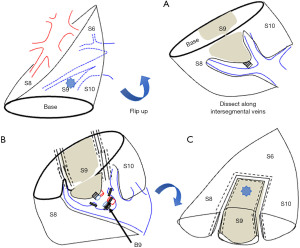
Right S9
Right S9 has three intersegmental planes and is usually narrow. Therefore, the resection of this area is planned as a combined resection with the neighboring S8 or S10 segment. The conventional method is to isolate the artery and bronchus from the fissure by dividing the intersegmental border between S6 and S8. Another approach is the vein-first which dissects the lung parenchyma via pulmonary ligament approach (11). If there are intersegmental veins, the hilar parenchymal incision along the vein will reach the bronchus, and we treat the artery after the bronchial division. If the vein is running along the bronchus, we divide the vein, then manage the bronchus and artery (12).
Right S10
The conventional method is to isolate the deeply located artery and bronchus from the interlobar fissure. Total division of the intersegmental border between S6 and the basal segment is one of the methods to make the procedure easier (13). However, the extra division between non-affected segments may be the demerit of this procedure. We usually take the vein-first approach incising the lung parenchyma along V6 and intersegmental V9+10, and reach to the bronchus and artery (13-15).
Left S1+2
We first expose the V1+2 at the mediastinal side of the lung, making parenchymal incision along the vein, then dissect A1+2ab cranial to the V1+2. We may preserve the intersegmental V1+2a and only divide the intrasegmental V1+2b depending on the character of the tumor and its location.
Left S3
We expose the V1+2 and V3 from the anterior side. The parenchymal incision along both veins and the division of intersegmental V3c exposes the A3 and make the encircling of this artery (Video 1). The A3 may be transected at both cranial or caudal to V1+2.
Left S4
We incise the lung parenchyma along V3 which runs between S3 and S4, and V4+5 which runs between S4 and S5. This makes the exposure of B4 smooth.
Left S5
We incise the lung parenchyma along V4+5 and get access to B5 anteriorly.
Left S6
Left S6 segmentectomy is similar to right segmentectomy. We chose the vein-first approach only when there is an incomplete fissure.
Left S8
We expose the inferior basal vein isolating the intra-segmental V8 and intersegmental vein between S8 and S9. The dissection along the vein which runs beside the B8 and B9 easily isolates and encircles the B8.
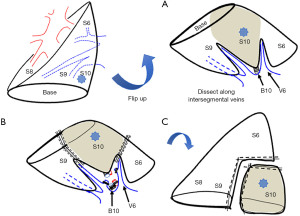
Left S9
Left S9 is mostly the same as right S9 segmentectomy. The vein-first, which dissects the lung parenchyma via the pulmonary ligament approach, is more accessible to reach the bronchus than the right S9 as there is little S7 (Figure 2, Video 1). The dissection of A9 depends on the completeness of the interlobar fissure and its relations with other structures (12).
Left S10 (Figure 3)
Left S10 is mostly the same as right S10 segmentectomy. The vein-first approach is more accessible to reach the bronchus than the right S10 for right-handed surgeons (13-15).
Other segmentectomies
Generally, the pulmonary venous wall is robust and tolerates stretching and longitudinal blunt dissection. For example, we sometimes bluntly dissect the lung parenchyma between left S3 and S4 along the V3a from interlobar fissure and open the intersegmental border between the upper division and lingula using staplers. This procedure enables the maneuver of B3a and A3a to remove some subsegments, preserving the connection of S3b and S4b without total division between the upper division and lingula. We can enter the vascular sheath of most PVs and easily incise lung parenchyma along them. However, V2c sometimes has many small branches and may be difficult to enter the vascular sheath in some cases.
In every segmentectomy or subsegmentectomy, we always observe the veins through thin-section CT and 3D-CT and discuss if some intersegmental veins can help dissection of the affected segment and help reach the hilar artery or bronchus. As we have described, we usually perform parenchymal dissection along the veins from the proximal side toward the peripheral side, but in some situations, we may do it in the opposite direction. For example, we present the representative case of this resection method.
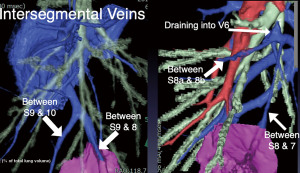
The patient was diagnosed with a nontuberculous mycobacterial infection. Preoperative CT revealed the right basal segmental bronchial branch indicating a common trunk of B8 and 9. The subsegmental bronchi, B8b and B9b, also created the common trunk. The intersegmental veins between S7 and 8, and S8a and S8b are aberrantly draining into an atypical branch of V6 (Figure 4). Based on these variant findings and planning by 3D-CT, we identified the intersegmental veins between S7-8 and S8a-8b during the operation. We made the parenchymal incision along these veins from the peripheral side toward the hilum, which led to the artery and bronchus of the target segment of S8b9b. After delineating the intersegmental plane by the inflation-deflation method, we deeply dissected the parenchyma along the inter-segmental vein between S9 and 10, dividing the vein draining S9b, and divided the intersegmental planes using staplers along the inflation-deflation lines, preserving the intersegmental vein (Video 2).
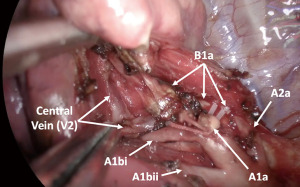
This dissection along the vein can also be applied to subsegmentectomies. For example, we expose the central vein between B1a and B1b before dividing B1a and dissect the inter-subsegmental plane using an energy device from the hilum to the periphery when we perform S1a subsegmentectomy of the right lung. The remaining connection of B1a anchors the target segment as counter traction and the B1a is finally divided after the sufficient peeling of the central vein and division of the parenchyma between S1a and S1b (Figure 5, Video 1).
Postoperative considerations and tasks
The chest tube was maintained until at least 4 hours postoperatively, and in recent years, if not hemorrhagic, it was removed when the air leak stopped, regardless of the amount of postoperative pleural effusion. We performed complete thoracoscopic anatomical segmentectomy in a limited number of cases in 2004 and started to perform segmentectomy with this approach in 2009, expanding the indication and standardizing it for all segmentectomy procedures. Therefore, we retrospectively reviewed the database of 425 patients who underwent thoracoscopic anatomical segmentectomy at our institutes between January 2009 and September 2023. The chest tube was removed on the first postoperative day in 306 cases (72.0%). Prolonged air leakage (7 or more days) was observed in 12 cases (2.8%). The median operative time was 169 minutes (interquartile range, 139–199 minutes), and the median amount of bleeding was 50 mL (interquartile range, 0–111 mL). Hospital and 30-day mortality were not observed.
Tips and pearls
PVs can be peeled inside its vascular sheath and can tolerate longitudinal blunt dissection. By dissecting tissue along the vein, the bronchus and artery can be encircled simultaneously, eliminating unnecessary manipulation. Intersegmental veins can serve as landmarks of the intersegmental plane, and the dissection along the inter-segmental vein facilitates the stapler to be fixed in the appropriate position.
Discussion
Lung segmentectomy is increasingly indicated not only for nonneoplastic disease but also for small lung cancer. Segmentectomy is performed by dissection and dividing the corresponding bronchi and pulmonary arteries at the pulmonary hilum; it distinguishes segmentectomy from wedge resection (16). This technique is technically more difficult than lobectomy because of the intricate and complex structure of the pulmonary hilum. In addition, the bronchi in each segment have many variations in branching patterns, and the arteriovenous branches are even more complicated, requiring a sufficient understanding of the anatomy of each case to perform an anatomic segmentectomy. Detailed assessment of CT is essential to understand the anatomy, and it is effective to observe the lungs from a surgeon’s view using 3D-CT in addition to thin-sliced CT. We have reported that the surgeon can magnify, de-magnify and rotate this 3D-CT depending on the lesion and that it is straightforward for surgical planning to limit its ROI to the lung lobe of the target segment and eliminate other parts of the lungs (5). During these efforts, we have shown that unconventional approaches can sometimes be effective in thoracoscopic procedures, and we began to focus on the utility of the vein-first approach, which we have used for many anatomical segmentectomies (12-15). It may be difficult to perform our method without 3D-CT reconstruction; however, thin-sliced views of sagittal and coronal angle may help grasp the intersegmental vein etc.
The term “vein-first” method for lung cancer has usually been used in an oncological aspect. Its advantage for lung lobectomy may be the improvement of the prognosis by reducing the number of circulating tumor cells into the peripheral circulation by blockade of venous return (17). However, the vein-first strategy for lung segmentectomy described here is focused only on the procedural aspects.
Unlike arteries, PVs are durable and can be peeled inside its vascular sheath, less fragile as it can be grasped with forceps, and can tolerate longitudinal blunt dissection as long as their small branches are not torn. In addition, PVs often run between segments and can serve as landmarks of the intersegmental plane if they are accurately identified using thin-sliced CT and 3D-CT reconstruction. When the PV runs along the bronchus, which drains the proper segment, the bronchus of the affected segment can be isolated after the division of the vein; thus, this method is also utilized in the resection of basal segments in other institutes (11,18,19). This approach is also valuable for robotic-assisted lung segmentectomy procedures, as the view and manipulation from the caudal side first encounter PV exposures in many cases. We have reported the learning curve for thoracoscopic individual basal segmentectomy, and indicated that the inflection point for the learning curve of thoracoscopic basal segmentectomy was reached after 42 cases (20), that was after ten cases done in the second period after the introduction of this vein-first approach in basal segmentectomies. We have used the vein-first approach for most segmental and subsegmental resections as it often exposes the artery and bronchus more straightforwardly, may preserve some original parenchyma, and makes the procedure simple; however, in patients with chronic emphysema, parenchymal incisions at the pulmonary hilum should be kept minimal. One of the statements of the recent European Society of Thoracic Surgeons expert consensus recommendations on technical standards of segmentectomy is “Except simple and clear anatomy, the control of the vein is best done within the parenchyma and not at the hilum level as a segmental vein can drain >1 segment” (16). Our method meets this consensus. Whether we divide or preserve the affected segments’ intersegmental veins depends on the lesions’ nature and distance.
The systemic ICG method has been used worldwide in recent years, and some segmentectomy methods may have changed. The vein-first strategy has two merits even in the ICG method era: (I) when the segmental artery is located deeply in the parenchyma, the precise assessment of hilar structure using 3D-CT, finding the neighboring vein, and dissection along the vein can lead to the target segmental artery. We demonstrated the representative case in Video 2. (II) When we use the systemic ICG method, we need to divide the intersegmental plane only by staplers. In our experience, segmentectomy using the ICG method is less precise around the hilar structures and tends to resect larger areas to avoid some mistakes. Recent developments of staples for thick tissue, such as black stapler cartridges, made it possible to catch and divide the thick tissue; however, it is sometimes still hard to hold the correct point as the opening angle is limited. It is also hard to keep the grasp point as it slips at the thicker hilum side. The dissection along the intersegmental vein makes the parenchyma thinner to apply staplers, and it also facilitates the stapler cartridge or anvil to be fixed in the appropriate position, and may prevent extra parenchymal convergences.
Limitations are as follows: In this report, the purpose of the term “vein-first” strategy is specific to segmentectomy techniques and does not consider other factors. For example, even small-sized non-small cell lung cancer requires hilar lymph node dissection when it is the invasive type, and some other different techniques or approaches may be considered depending on the resection area and the location of the lymph nodes. In this regard, it is advisable to choose a method based on the pathological characteristics of each patient.
Conclusions
When we precisely grasp the anatomy by 3D-CT, the vein-first strategy for lung segmentectomy sometimes merits the operation, avoiding unnecessary manipulations. Hilar parenchymal dissection along the intersegmental vein facilitates the stapler being fixed in the appropriate position, may prevent extra parenchymal convergences, may preserve the original lung shape in basal segmentectomy, and so on.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://ccts.amegroups.com/article/view/10.21037/ccts-23-9/rc
Peer Review File: Available at https://ccts.amegroups.com/article/view/10.21037/ccts-23-9/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts-23-9/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Data collection and analyses were approved by the ethics committee of the Faculty of Medicine, Yamagata University (IRB No. 2023-259). Written informed consent was obtained from the patient for publication of this article and the accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Altorki N, Wang X, Kozono D, et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N Engl J Med 2023;388:489-98. [Crossref] [PubMed]
- Nomori H, Okada M. Illustrated anatomical segmentectomy for lung cancer. Tokyo: Springer; 2012.
- Oizumi H, Endoh M, Takeda S, et al. Anatomical lung segmentectomy simulated by computed tomographic angiography. Ann Thorac Surg 2010;90:1382-3. [Crossref] [PubMed]
- Oizumi H, Kanauchi N, Kato H, et al. Anatomic thoracoscopic pulmonary segmentectomy under 3-dimensional multidetector computed tomography simulation: a report of 52 consecutive cases. J Thorac Cardiovasc Surg 2011;141:678-82. [Crossref] [PubMed]
- Okada M, Mimura T, Ikegaki J, et al. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007;133:753-8. [Crossref] [PubMed]
- Endoh M, Oizumi H, Kato H, et al. How to demarcate intersegmental plane with resected-segments inflation method using the slip knot technique in thoracoscopic anatomic segmentectomy. J Vis Surg 2017;3:100. [Crossref] [PubMed]
- Yao F, Wu W, Zhu Q, et al. Thoracoscopic Pulmonary Segmentectomy With Collateral Ventilation Method. Ann Thorac Surg 2021;112:1814-23. [Crossref] [PubMed]
- Misaki N, Chang SS, Igai H, et al. New clinically applicable method for visualizing adjacent lung segments using an infrared thoracoscopy system. J Thorac Cardiovasc Surg 2010;140:752-6. [Crossref] [PubMed]
- Mun M, Nakao M, Matsuura Y, et al. Novel techniques for video-assisted thoracoscopic surgery segmentectomy. J Thorac Dis 2018;10:S1671-6. [Crossref] [PubMed]
- Kikkawa T, Kanzaki M, Isaka T, et al. Complete thoracoscopic S9 or S10 segmentectomy through a pulmonary ligament approach. J Thorac Cardiovasc Surg 2015;149:937-9. [Crossref] [PubMed]
- Suzuki K, Oizumi H, Suzuki J, et al. Thoracoscopic Lateral Basal Segmentectomy Based on Dissection Along the Intersegmental Veins. Ann Thorac Surg 2023;115:e83-5. [Crossref] [PubMed]
- Oizumi H, Kato H, Suzuki J, et al. Thoracoscopic anatomical S10 segmentectomy: a posterior approach. J Vis Surg 2018;4:142-5. [Crossref]
- Endoh M, Oizumi H, Kato H, et al. Posterior approach to thoracoscopic pulmonary segmentectomy of the dorsal basal segment: A single-institute retrospective review. J Thorac Cardiovasc Surg 2017;154:1432-9. [Crossref] [PubMed]
- Takamori S, Oizumi H, Suzuki J, et al. Thoracoscopic anatomical individual basilar segmentectomy. Eur J Cardiothorac Surg 2022;62:ezab509. [Crossref] [PubMed]
- Brunelli A, Decaluwe H, Gonzalez M, et al. European Society of Thoracic Surgeons expert consensus recommendations on technical standards of segmentectomy for primary lung cancer. Eur J Cardiothorac Surg 2023;63:ezad224. [Crossref] [PubMed]
- Wei S, Guo C, He J, et al. Effect of Vein-First vs Artery-First Surgical Technique on Circulating Tumor Cells and Survival in Patients With Non-Small Cell Lung Cancer: A Randomized Clinical Trial and Registry-Based Propensity Score Matching Analysis. JAMA Surg 2019;154:e190972. [Crossref] [PubMed]
- Pu Q, Liu C, Guo C, et al. Stem-Branch: A Novel Method for Tracking the Anatomy During Thoracoscopic S9-10 Segmentectomy. Ann Thorac Surg 2019;108:e333-5. [Crossref] [PubMed]
- Zhu Y, Pu Q, Liu C, et al. Trans-Inferior-Pulmonary-Ligament Single-Direction Thoracoscopic RS9 Segmentectomy: Application of Stem-Branch Method for Tracking Anatomy. Ann Surg Oncol 2020;27:3092-3. [Crossref] [PubMed]
- Takamori S, Oizumi H, Suzuki J, et al. Learning Curve for Thoracoscopic Individual Basilar Segmentectomy: 18-Year Experience. World J Surg 2023;47:2917-24. [Crossref] [PubMed]
Cite this article as: Oizumi H, Sasage T, Takamori S, Suzuki J, Watanabe H, Kato H. Vein-first strategy for thoracoscopic lung segmentectomy under use of three-dimensional reconstruction of computed tomography. Curr Chall Thorac Surg 2024;6:1.