
Localization approaches to improve surgical resection of peripheral pulmonary lesions: a clinical practice review
Introduction
Computed tomography (CT) is an important diagnostic modality in both the workup and differentiation of pulmonary and thoracic pathology. With the results of randomized control trials showing a reduced lung cancer mortality with CT chest screening (1,2) and the United States Preventive Services Task Force recommendations for serial low-dose CT scans in high-risk individuals (3), the proliferation of CT chest imaging has continued to accelerate. A byproduct of this growing utilization has been an increased incidence of indeterminate peripheral pulmonary lesions (PPLs). An analysis of one large, integrated health system showed that from 2006 to 2012 the annual rate of pulmonary nodule identification increased from 3.9 to 6.6 per 1,000 person-years (4). When this value was applied to the general United States population, it was estimated that more than 1.5 million pulmonary nodules would be identified yearly, a value ten times greater than previously believed (4).
Once PPLs are identified, challenges from both a diagnostic and management perspective exist. Fortunately, calculators and guidelines exist to help assess risk. The pretest probability of malignancy can be determined by clinical intuition and/or validated prediction calculators (5,6). Calculators have been shown to offer significant diagnostic utility when trying to determine whether pulmonary nodules are malignant or benign (7) and improve physician management (8). Similarly, guidelines exist to assist with initial and follow up management of incidentally found nodules (9,10). However, physician adherence to these recommendations is variable (11).
Although the combination of CT imaging with risk calculators and guidelines can provide meaningful information regarding the risk of malignancy for PPLs, tissue biopsy is still needed in many cases. If a tissue diagnosis is required, guidelines recommend consideration of CT-guided biopsies, bronchoscopic biopsies, or surgical resection (9). While early recognition and phenotyping of lung cancer is important from a treatment perspective, excision of PPLs may be technically challenging from a surgical perspective. Nodules that are not amenable to direct visualization or palpation often require excess tissue removal to ensure appropriate resection of the concerning lesion or conversion from a minimally invasive approach to a more invasive open surgery (12-14). While several strategies exist currently to improve identification of thoracoscopically “invisible” nodules, evidence suggests that placement of markers facilitates improved visualization of PPLs during surgery (15-17).
In this paper, we hope to provide a brief overview of the current landscape regarding preoperative marking of PPLs, providing a summary of localization techniques, current literature, and perhaps, most importantly, possible steps forward.
Indication for PPL localization techniques
A critical aspect of any surgery is determining which structures should undergo resection and which structures must be preserved. Historically, surgeons have relied on manual palpation and visual examination to achieve this result (18). Despite best efforts to ensure appropriate removal of the desired target and clear margins, suboptimal outcomes do occur. Studies have shown that tumor margins of less than 1 cm are a significant risk factor for local recurrence in early-stage surgical lung cancer after limited resection (19). In contrast, a larger tumor margin distance is associated with lower risk of lung cancer recurrence and longer overall survival in patients undergoing wedge resections for non-small cell lung cancers less than or equal to 2 cm in size (20). Similar findings regarding margin length have been seen for the risk of recurrence in surgically resected metastatic lung lesions (21). Additionally, there exists the potential for injury to pivotal vascular structures. While the incidence of major vascular injuries during minimally invasive thoracic surgery is rare (22,23), its occurrence may increase the risk of morbidity and mortality due to longer operating time, further lung manipulation, an increased risk of damage to adjacent tissues, and an increased blood loss (24).
In cases of PPLs suspected of representing, or confirmed to be, early-stage lung cancer, recent studies have shown comparable results for lung sparing surgery versus lobectomy. For stage T1a–bN0 (<2 cm in size, node negative) tumors, sublobar resection was comparable to lobectomy with respect to disease-free survival (25), had less postoperative complications (26), and improved post-operative measures of quality of life (27). For patients undergoing segmentectomy, 5-year overall survival was actually superior (28). Given these comparable to improved survival results and the improved perioperative mortality, morbidity, and median length of hospital stay, there is an increasing emphasis on performing minimally invasive, lung sparing surgery (29).
To facilitate this process, attempts are made prior to surgery to precisely pinpoint the location of PPLs. Various methods have been developed for localizing PPLs that usually rely on the placement of metallic markers or the injection of liquid dyes (30-32). While the effectiveness and safety of these methods are variable (30-32), an optimal localization technique would facilitate definitive resection of the target while preventing extensive resection of non-involved lung parenchyma. It should exhibit a high accuracy rate and minimal risk of procedural complications, be completed in a reasonable amount of time, last long enough to provide adequate time between the localization procedure and surgery, allow access to the entire lung parenchyma, and be readily available at institutions without a significant cost-increase.
Studies have shown that the failure to localize a PPL is related to the distance between the PPL and the closest pleural surface. A retrospective study showed that the main reason for conversion from video-assisted thoracoscopic surgery (VATS) to a thoracotomy was due to inability to localize the lesion (33). Univariate and multivariate analyses showed that distance to the nearest pleural surface was the one significant risk factor that led to failure to detect the nodules with a 63% failure rate for lesions <10 mm in size located more than >5 mm from the pleural surface (33).
Ground-glass lesions, which are being detected with increasing prevalence on outpatient CT scans (34), represent another challenge for resection as these lesions are not visible or palpable. A randomized control trial showed that preoperative marking of PPLs increases the diagnostic yield of initial VATS wedge resection without the need for thoracotomy, decreased operative time to nodule excision, and reduced stapler firings (35). Thus, utilization of localization techniques prior to VATS for ground-glass lesions, subcentimeter nodules, and lesions greater than 1cm from the pleura has become more commonplace at equipped facilities (30-32).
Types of PPL localization techniques
Preoperative localization of PPLs necessitates the use of image guidance, traditionally involving CT, and can be broadly categorized into two major types. The first type of localization technique involves the placement of a metallic marker, such as hook wires, micro-coils, and fiducial markers. The second approach is to inject a liquid material such as methylene blue (MB), India ink, barium, lipiodol, indocyanine green (ICG), or radionuclides via a fine needle.
Metallic markers
The original PPL localization technique is the image-guided hook wire localization. Commonly used in breast lesion localization since the 1970s, the hook wire approach was first used in the lung in 1992 to percutaneously mark PPLs (36) and has become a widely utilized strategy since (30) (Table 1). While boasting high procedural accuracy for PPL localization of 90% or greater (30,37,38) and mechanical simplicity that negates the need for intraoperative fluoroscopy, this technique has the potential for several complications. Risk of dislodgement prior to resection is increased when the hook wire is located proximal to the pleura (38), with an incidence ranging from 2.4% to 6.9% (30). Additionally, an increasing amount of length of the hook wire placed within the lung parenchyma is correlated with an increased risk of hemorrhage (39), occurring in 13.9% to 35% of cases (30). Other complications include pneumothorax, post-resection hook wire retention and rarely, air embolism (16,40).
Table 1
| Localization technique | Pros | Cons | References |
|---|---|---|---|
| Metallic | |||
| Hook wire localization | High procedural accuracy (90% or greater) | Risk of dislodgement prior to resection, especially when located proximal to the pleura (2.4% to 6.9% incidence); Increased risk of hemorrhage with more hook wire length within lung parenchyma (13.9% to 35% incidence) | Park et al. (16), Lin et al. (30), Mack et al. (36), Miyoshi et al. (37), Zhao et al. (38), Zhang et al. (39), Tang et al. (40) |
| Microcoil localization | Decreased need for thoracotomy or VATS anatomic resection; comparable success rates to hook wire with fewer complications | Requires fluoroscopic guidance during surgery | Park et al. (16), Finley et al. (35), Velasquez et al. (41) |
| Fiducial marker localization | High success rates (95% or greater); various types allowing flexibility in surgical technique; allows surgery at later date or institution | Requires fluoroscopy for visualization, risk of complications: pneumothorax, hemorrhage, vascular embolization, migration, depending on type used | Lin et al. (30), Velasquez et al. (41), Sharma et al. (42), Sancheti et al. (43), Anantham et al. (44), Casutt et al. (45) |
| Dye markers | |||
| MB injection | Relatively safe, cost-effective, and no additional visualization equipment required; high PPL identification rates (usually >90%) | Localization must be same day as surgery to prevent dispersion (up to 8% failure rate after 3-hour delay); potential interference from anthracotic pigment | Vandoni et al. (46), Lenglinger et al. (47), McConnell et al. (48), Nomori et al. (49) |
| Contrast media localization | High success rates with fluoroscopic guidance during resection; lipiodol retained up to 3 months, preferred over barium for time delay cases | Lipiodol poses embolism risk if accidentally injected into bloodstream; barium induces inflammatory response making histological interpretation challenging at times | Lin et al. (30), McDermott et al. (31), Moon et al. (50), Watanabe et al. (51), Chella et al. (52), Bellomi et al. (53), Ambrogi et al. (54), Galetta et al. (55), Sortini et al. (56) |
| Gamma-emitting radioisotope localization | Effective localization with counter positioning during resection | Surgery must be within 24 hours to maximize isotope effect; requires additional equipment, cost, radiation exposure | Lin et al. (30), McDermott et al. (31), Chella et al. (52), Bellomi et al. (53), Ambrogi et al. (54), Galetta et al. (55), Sortini et al. (56) |
| ICG marking | Utilizes near infrared fluorescence for visualization during resection | Potential accumulation in other organs with systemic injection; inhalational route has both feasibility and accessibility limitations | Abbas et al. (57), Anayama et al. (58), Rho et al. (59), Li et al. (60), Okusanya et al. (61), Quan et al. (62), Wang et al. (63) |
| Emerging techniques | |||
| Intraoperative molecular imaging | Quick binding to target receptor; favorable tumor visualization | Limited validation data; long term effectiveness and safety require further research | Zhang et al. (64), Schouw et al. (65), Sarkaria et al. (66) |
PPL, peripheral pulmonary lesion; VATS, video-assisted thoracoscopic surgery; MB, methylene blue; ICG, indocyanine green.
Microcoils are also utilized to mark PPLs. These metallic markers are placed percutaneously with imaging guidance with the distal end in or near the PPL of interest and the proximal end coiled in the pleural space to allow resection of the tumor and coil together with fluoroscopic guidance during surgery (35,41). Unlike the hook wire approach, no portion of the microcoil is visible outside of the patient. Initial studies showed that microcoil localization decreased the need for thoracotomy or VATS anatomic resection for the diagnosis of PPLs (35). Meta-analysis has shown comparable success rates for localization with microcoil compared to hook wire and less incidence of complications, including pneumothorax and pulmonary hemorrhage, suggesting that microcoils may be a safer, yet equally effective localization strategy (16).
In contrast to other metallic markers, fiducial markers are placed either percutaneously or via bronchoscopy within or near the PPL of interest with success rates of 95% or greater (30,41-44). Similarly to microcoils, fluoroscopy is needed to visualize fiducial markers and the entirety of the marker is placed within the patient. Various forms of fiducial markers exist, including linear, coil-tailed, coil-spring, and two-band fiducial markers (45). An advantage of fiducial markers is that they do not require the surgeon to approach the PPL via the path that the marker was placed in, thus allowing for flexibility in the surgical technique (42). Also, surgery may be performed at a later date or at a different institution based on logistical constraints. While pneumothorax, hemorrhage, vascular embolization, or migration can occur (30,41-44), it appears that the rate of migration may depend on the type of fiducial marker used, with data showing that the coil-tailed and coil-spring markers having the lowest migration rate, and thus providing some rationale for their preferential use, if available (45).
Dye markers
The principle behind MB injection is to stain the pleura above the nodule, allowing the surgeon to trace the blue tract to the target (46). MB is widely used in clinical practice as it is relatively safe, cost-effective, and does not require additional visualization equipment, such as fluoroscopy, during resection as no foreign body is implanted in the parenchyma or pleura (46,47). Patients tolerate the procedure well as there is no implantation of foreign bodies. PPL identification rates after MB placement are usually greater than 90% (30,41,46,47). However, a significant limitation to MB use is the necessity to perform localization on the same day as surgical resection, ideally within 2 to 3 hours of surgery, to prevent dye dispersion (47,48). Delays of 3 hours or more after MB injection appear to substantially impact the ability to localize the target PPL, with a failure rate of up to 8% (46). Attempts to prevent dispersion utilized mixtures of MB stained with autologous blood, contrast agents, collagen, or stained glue (48,49). Additionally, the presence of anthracotic pigment on the pleural surface may interfere with proper localization due to its similar appearance to the MB-stained PPL (47).
Contrast media, such as barium or lipiodol, may also be utilized for PPL localization through percutaneous or bronchoscopic methods. Using fluoroscopic guidance for visualization during surgical resection, nodules are located with a high degree or success (30,31,50,51). Because lipiodol can be retained in lung tissue for up to 3 months and is less likely to induce an inflammatory response seen with barium that may make histological interpretation of the resected PPL lesion more challenging, it is often preferred, especially when there is a time delay between marking and surgery (50,51). Although these properties of lipiodol are beneficial, it is water insoluble and accidental injection into bloodstream poses a risk of embolism, cerebrovascular accidents, and lymphatic obstruction (51).
Gamma-emitting radioisotope like technetium 99 may also be used to localize PPLs. Placed percutaneously with CT guidance, radioisotopes are detected using a counter that can be positioned in multiple angles around the targeted lesion to identify it during surgical resection (52). Similarly to MB, timing of the subsequent surgical resection is important as it should be coordinated to occur within 24 hours to maximize the effect of the isotope (30,31,52-56). Limitations to this localization approach include the additional equipment required and associated cost as well radiation exposure (52-56).
ICG marking of PPLs utilizes near-infrared fluorescence during surgical resection to visualize the target area. The procedure can be performed bronchoscopically (57,58) or percutaneously (58-60) using a mixture of ICG with another vehicle such as lipiodol (58,59) or MB (57). Although it can also be utilized to mark lesions through systemic injection, this approach is limited by potential for accumulation of ICG in other organs (61). Recent studies have also utilized an inhalational route for ICG to mark tumors and facilitate surgical resection with promising results (62,63).
Targeted optical imaging agents
In addition to the above non-targeted PPL marking modalities, other localization techniques exist including intraoperative molecular imaging utilizing targeted optical imaging agents. Targeted tracers are composed of a carrier molecule, such as an antibody, peptide or small molecule, with a fluorescent probe attached to it and are directed at a specific disease biomarker to allow for increased specificity in accumulating in the target tissue (64,65). In a preliminary validation study, the use of pafolacianine has revealed promising results, showing its quick binding to the target receptor and swift clearance from healthy non-cancerous tissues, making it suitable for infusion either the day before or on the day of the surgery (66). Other similarly designed agents are in different stages of development. It will be interesting to see the impact of these agents on the field as more data emerges in the future.
Percutaneous versus bronchoscopic guided localization
Once a decision on a dye or metallic marker is confirmed, the modality used to deploy the marker must be chosen. Although percutaneous localization with CT guidance has traditionally been the norm, there is mounting evidence supporting the adoption of bronchoscopic approaches. CT-guided lung biopsies offer superior diagnostic yield compared to traditional bronchoscopic modalities (67). However, this approach is limited by an increased risk for complications. Meta analyses have shown that the incidence of pneumothorax post CT-guided biopsy ranges from 15% to 25% with ~7% requiring chest tube drainage (68-70). The incidence of hemorrhage ranged from 3% to 7% (64,65). Additionally, biopsy of smaller lung nodules is also more likely to result in pneumothorax (70) and less likely to be diagnostically accurate (71,72).
Recent advances in bronchoscopic technology utilizing robotic-assisted bronchoscopy with shape sensing technology (73-77) and electromagnetic navigation with digital tomosynthesis (78-81) have allowed for improved diagnostic yield compared to prior bronchoscopic approaches with minimal procedural complications. Additionally, studies have shown that bronchoscopic marking has a favorable safety profile compared to CT-guided biopsy with comparable effectiveness (17,82). Because it can be performed in the operating room, bronchoscopic marking may also reduce the time between localization and surgery (83).
An additional advantage of bronchoscopic marking is the ability to access several anatomic locations that are often not amenable to the percutaneous approach, including the apex and interlobar (17). Multiple nodules or deeper nodules (more than 3 cm from the pleural surface) may be marked during the same procedure without substantially increasing the procedural complication risks (17,70). Finally, there is the ability to biopsy and mark the target lesion while also staging the mediastinal and hilar lymph nodes in one procedure, thus negating the need for a second anesthetic event.
Consideration of which nodules should be marked
Presently, there are no clear consensus guidelines or recommendations on which nodules should be marked. Suzuki et al. suggested that nodules less than 1cm in diameter and at a depth of >5 mm from the pleural surface should be preoperatively marked based on the failure rate to detect these types of lesions intraoperatively was 63% (33). Similarly, Tamura et al. recommended that solid lung nodules <15 mm in diameter and at a depth to the nearest pleural surface >10 mm should be preoperatively marked (84). Interestingly, these authors also noted that there is almost no possibility to detect non-solid nodules at a pleural depth greater than 3 mm, independent of size or diameter, suggesting that, potentially, all ground-glass nodules should be marked prior to resection (84).
Based on the above literature, combined with our own institutional experience performing marking procedures, we recommend considering the following criteria for the evaluation of PPLs for localization and their subsequent marking procedure.
- Selection:
- Newly identified solid pulmonary nodules <1 cm in size and/or >0.5 cm from the pleural surface;
- Pure ground-glass opacity (GGO) pulmonary nodules regardless of size;
- Procedure:
- Injection of 0.5 mL of biocompatible fluorescent and optically visible dyes no more than 1 cm from the pleural surface after traversing the nodule with the needle;
- Placement of a single, large fiducial marker near and medial to the nodule;
- Assessment:
- Marking procedure:
- Utilization of cross-sectional imaging [CT, cone-beam CT, or three-dimensional (3D) mobile fluoroscopy] to ensure appropriate placement of radiopaque markers (if used) in relation to the target lesion;
- Surgery:
- Visualization of the visceral pleural surface without broad dye dispersion or dye on the parietal pleural surface;
- Palpation and/or radiographic/ultrasonographic visualization of fiducial marker placed in and around the nodule;
- At least 1 cm margins on final pathology.
- Marking procedure:
Formal guidelines will also be needed to assess PPL localization success. Traditionally, PPL localization has been judged subjectively by the operator or by the surgeon at the time of resection. Additional metrics may include a post-localization CT chest or intraoperative 3D imaging afforded by cone beam CT or mobile 3D fluoroscopy units.
A clear follow up plan to evaluate if minimal lung tissue was resected after PPL marking would also be informative. Because a minimally invasive surgical approach leads to less impaired pulmonary function tests (PFTs) values and improved 6-minute walk distances (85,86), we suggest a comparison of baseline and post-resection PFTs be routinely performed to further understand if lesion marking facilitates improved patient outcomes. This evaluation may allow for a rough extrapolation of how much lung function and tissue may be saved by preventing a larger surgical resection. Efforts to characterize this potential benefit will require dedicated future research studies to determine if there are meaningful improvements in both objective, based on PFTs and walk test values, and subjective, based on patient quality of life surveys, measures.
Combination marking: a new approach
As discussed, bronchosocpic PPL marking has a favorable risk profile and comparable efficacy to percutaneous approaches. Available evidence suggests that both dye and fiducial markers are reasonable choices for preoperative localization prior to surgery. Data also suggests that utilizing both techniques together may have a positive impact on the marking process by allowing them to act together in an almost synergistic fashion. Previous studies utilizing a dual localization of a hook wire combined with a radiotracer (87), with lipiodol (88), or with ICG and lipiodol (89) have shown excellent results in terms of ability to successfully mark the PPL and facilitate surgical resection without significant increase in procedural time.
A recent study showed that placing an ICG soaked fiducial marker to localize PPLs allowed for appropriate localization of the lesion and a delayed surgical excision up to 9 days after marker placement (90) (Figure 1). The fiducial marker operates both as a fluoroscopic marker while also acting as a local binder, holding the dye hyperlocal and preventing unwanted peri-lesional leak. This composite effect allows both for increased time between PPL localization and surgery and also provides the thoracic surgeon more options intraoperatively to locate the target lesion. While more research is needed, bronchoscopic placement of dye-soaked fiducial markers may be an important contributor in facilitating a minimally invasive lung sparing surgery.
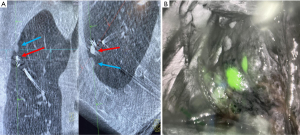
Conclusions
With the advent of lung cancer screening and increased CT chest utilization, the incidence of PPLs being detected and referred for surgical resection will continue to increase. While emerging data has been helpful in identifying and selecting the appropriate patients and lung nodules for different types of surgical approaches, this field will continue to evolve as more data becomes available. Due to the decreased morbidity and recovery time with minimally invasive thoracic surgery, this approach will continue to be used to simultaneously establish a diagnosis and definitive treatment in many cases. Localization of PPLs, whether with metallic or liquid markers or both together via CT guidance or bronchoscopic modalities, has been shown via numerous single-center studies and meta-analyses to be a safe and successful method to facilitate lung sparing surgery. No specific approach to marking PPLs has been shown to be superior at this time, although this may reflect a lack of randomized data and multiple products or modalities being available at institutions. Similarly, standardized definitions of successful PPL marking are lacking. Further studies and development of guidelines are needed to identify appropriate patients with PPLs for localization procedure, to develop criteria for successful marking procedures, and to ensure procedures are done in a standardized fashion to optimize patient care.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://ccts.amegroups.com/article/view/10.21037/ccts-23-25/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts-23-25/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All clinical procedures described in this study were performed in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for the publication of this article and accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med 2020;382:503-13. [Crossref] [PubMed]
- US Preventive Services Task Force. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2021;325:962-70. [Crossref] [PubMed]
- Gould MK, Tang T, Liu IL, et al. Recent Trends in the Identification of Incidental Pulmonary Nodules. Am J Respir Crit Care Med 2015;192:1208-14. [Crossref] [PubMed]
- Swensen SJ, Silverstein MD, Ilstrup DM, et al. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med 1997;157:849-55. [Crossref] [PubMed]
- McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013;369:910-9. [Crossref] [PubMed]
- Chen G, Bai T, Wen LJ, et al. Predictive model for the probability of malignancy in solitary pulmonary nodules: a meta-analysis. J Cardiothorac Surg 2022;17:102. [Crossref] [PubMed]
- Swensen SJ, Silverstein MD, Edell ES, et al. Solitary pulmonary nodules: clinical prediction model versus physicians. Mayo Clin Proc 1999;74:319-29. [Crossref] [PubMed]
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-e120S.
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology 2017;284:228-43. [Crossref] [PubMed]
- Tanner NT, Aggarwal J, Gould MK, et al. Management of Pulmonary Nodules by Community Pulmonologists: A Multicenter Observational Study. Chest 2015;148:1405-14. [Crossref] [PubMed]
- Allen MS, Deschamps C, Lee RE, et al. Video-assisted thoracoscopic stapled wedge excision for indeterminate pulmonary nodules. J Thorac Cardiovasc Surg 1993;106:1048-52. [Crossref] [PubMed]
- Murasugi M, Onuki T, Ikeda T, et al. The role of video-assisted thoracoscopic surgery in the diagnosis of the small peripheral pulmonary nodule. Surg Endosc 2001;15:734-6. [Crossref] [PubMed]
- Hirai S, Hamanaka Y, Mitsui N, et al. Role of video-assisted thoracic surgery for the diagnosis of indeterminate pulmonary nodule. Ann Thorac Cardiovasc Surg 2006;12:388-92. [PubMed]
- Isaka T, Takahashi K, Maehara T, et al. Intraoperative core needle biopsy under complete video-assisted thoracic surgery for indeterminate tumor of lung. Surg Endosc 2015;29:3579-87. [Crossref] [PubMed]
- Park CH, Han K, Hur J, et al. Comparative Effectiveness and Safety of Preoperative Lung Localization for Pulmonary Nodules: A Systematic Review and Meta-analysis. Chest 2017;151:316-28. [Crossref] [PubMed]
- Yanagiya M, Kawahara T, Ueda K, et al. A meta-analysis of preoperative bronchoscopic marking for pulmonary nodules. Eur J Cardiothorac Surg 2020;58:40-50. [Crossref] [PubMed]
- Alifano M. Invited commentary. Ann Thorac Surg 2013;96:1208. [Crossref] [PubMed]
- Masai K, Sakurai H, Sukeda A, et al. Prognostic Impact of Margin Distance and Tumor Spread Through Air Spaces in Limited Resection for Primary Lung Cancer. J Thorac Oncol 2017;12:1788-97. [Crossref] [PubMed]
- Wolf AS, Swanson SJ, Yip R, et al. The Impact of Margins on Outcomes After Wedge Resection for Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2017;104:1171-8. [Crossref] [PubMed]
- Nelson DB, Tayob N, Mitchell KG, et al. Surgical margins and risk of local recurrence after wedge resection of colorectal pulmonary metastases. J Thorac Cardiovasc Surg 2019;157:1648-55. [Crossref] [PubMed]
- Cerfolio RJ, Bess KM, Wei B, et al. Incidence, Results, and Our Current Intraoperative Technique to Control Major Vascular Injuries During Minimally Invasive Robotic Thoracic Surgery. Ann Thorac Surg 2016;102:394-9. [Crossref] [PubMed]
- Decaluwe H, Petersen RH, Hansen H, et al. Major intraoperative complications during video-assisted thoracoscopic anatomical lung resections: an intention-to-treat analysis. Eur J Cardiothorac Surg 2015;48:588-98; discussion 599. [Crossref] [PubMed]
- Bertolaccini L, Calabrese F, Brandolini J, et al. Vascular injuries during VATS lobectomies: keep calm, compress and have a plan. Ann Transl Med 2019;7:19. [Crossref] [PubMed]
- Altorki N, Wang X, Kozono D, et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N Engl J Med 2023;388:489-98. [Crossref] [PubMed]
- Kamel MK, Lee B, Harrison SW, et al. Sublobar resection is comparable to lobectomy for screen-detected lung cancer. J Thorac Cardiovasc Surg 2022;163:1907-15. [Crossref] [PubMed]
- Jiang S, Wang B, Zhang M, et al. Quality of life after lung cancer surgery: sublobar resection versus lobectomy. BMC Surg 2023;23:353. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Rusch VW. Initiating the Era of "Precision" Lung Cancer Surgery. N Engl J Med 2023;388:557-8. [Crossref] [PubMed]
- Lin MW, Chen JS. Image-guided techniques for localizing pulmonary nodules in thoracoscopic surgery. J Thorac Dis 2016;8:S749-55. [Crossref] [PubMed]
- McDermott S, Fintelmann FJ, Bierhals AJ, et al. Image-guided Preoperative Localization of Pulmonary Nodules for Video-assisted and Robotically Assisted Surgery. Radiographics 2019;39:1264-79. [Crossref] [PubMed]
- Cornella KN, Repper DC, Palafox BA, et al. A Surgeon's Guide for Various Lung Nodule Localization Techniques and the Newest Technologies. Innovations (Phila) 2021;16:26-33. [Crossref] [PubMed]
- Suzuki K, Nagai K, Yoshida J, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest 1999;115:563-8. [Crossref] [PubMed]
- Woodard GA, Udelsman BV, Prince SR, et al. Brief Report: Increasing Prevalence of Ground-Glass Nodules and Semisolid Lung Lesions on Outpatient Chest Computed Tomography Scans. JTO Clin Res Rep 2023;4:100583. [Crossref] [PubMed]
- Finley RJ, Mayo JR, Grant K, et al. Preoperative computed tomography-guided microcoil localization of small peripheral pulmonary nodules: a prospective randomized controlled trial. J Thorac Cardiovasc Surg 2015;149:26-31. [Crossref] [PubMed]
- Mack MJ, Gordon MJ, Postma TW, et al. Percutaneous localization of pulmonary nodules for thoracoscopic lung resection. Ann Thorac Surg 1992;53:1123-4. [Crossref] [PubMed]
- Miyoshi K, Toyooka S, Gobara H, et al. Clinical outcomes of short hook wire and suture marking system in thoracoscopic resection for pulmonary nodules. Eur J Cardiothorac Surg 2009;36:378-82. [Crossref] [PubMed]
- Zhao G, Yu X, Chen W, et al. Computed tomography-guided preoperative semi-rigid hook-wire localization of small pulmonary nodules: 74 cases report. J Cardiothorac Surg 2019;14:149. [Crossref] [PubMed]
- Zhang H, Li Y, Chen X, et al. Comparison of hook-wire and medical glue for CT-guided preoperative localization of pulmonary nodules. Front Oncol 2022;12:922573. [Crossref] [PubMed]
- Tang L, Zhang Y, Wang Y. Intraoperative identification of pulmonary nodules during minimally invasive thoracic surgery: a narrative review. Quant Imaging Med Surg 2022;12:5271-87. [Crossref] [PubMed]
- Velasquez R, Martin A, Abu Hishmeh M, et al. Placement of markers to assist minimally invasive resection of peripheral lung lesions. Ann Transl Med 2019;7:360. [Crossref] [PubMed]
- Sharma A, McDermott S, Mathisen DJ, et al. Preoperative Localization of Lung Nodules With Fiducial Markers: Feasibility and Technical Considerations. Ann Thorac Surg 2017;103:1114-20. [Crossref] [PubMed]
- Sancheti MS, Lee R, Ahmed SU, et al. Percutaneous fiducial localization for thoracoscopic wedge resection of small pulmonary nodules. Ann Thorac Surg 2014;97:1914-8; discussion 1919. [Crossref] [PubMed]
- Anantham D, Feller-Kopman D, Shanmugham LN, et al. Electromagnetic navigation bronchoscopy-guided fiducial placement for robotic stereotactic radiosurgery of lung tumors: a feasibility study. Chest 2007;132:930-5. [Crossref] [PubMed]
- Casutt A, Kinj R, Ozsahin EM, et al. Fiducial markers for stereotactic lung radiation therapy: review of the transthoracic, endovascular and endobronchial approaches. Eur Respir Rev 2022;31:210149. [Crossref] [PubMed]
- Vandoni RE, Cuttat JF, Wicky S, et al. CT-guided methylene-blue labelling before thoracoscopic resection of pulmonary nodules. Eur J Cardiothorac Surg 1998;14:265-70. [Crossref] [PubMed]
- Lenglinger FX, Schwarz CD, Artmann W. Localization of pulmonary nodules before thoracoscopic surgery: value of percutaneous staining with methylene blue. AJR Am J Roentgenol 1994;163:297-300. [Crossref] [PubMed]
- McConnell PI, Feola GP, Meyers RL. Methylene blue-stained autologous blood for needle localization and thoracoscopic resection of deep pulmonary nodules. J Pediatr Surg 2002;37:1729-31. [Crossref] [PubMed]
- Nomori H, Horio H. Colored collagen is a long-lasting point marker for small pulmonary nodules in thoracoscopic operations. Ann Thorac Surg 1996;61:1070-3. [Crossref] [PubMed]
- Moon SW, Wang YP, Jo KH, et al. Fluoroscopy-aided thoracoscopic resection of pulmonary nodule localized with contrast media. Ann Thorac Surg 1999;68:1815-20. [Crossref] [PubMed]
- Watanabe K, Nomori H, Ohtsuka T, et al. Usefulness and complications of computed tomography-guided lipiodol marking for fluoroscopy-assisted thoracoscopic resection of small pulmonary nodules: experience with 174 nodules. J Thorac Cardiovasc Surg 2006;132:320-4. [Crossref] [PubMed]
- Chella A, Lucchi M, Ambrogi MC, et al. A pilot study of the role of TC-99 radionuclide in localization of pulmonary nodular lesions for thoracoscopic resection. Eur J Cardiothorac Surg 2000;18:17-21. [Crossref] [PubMed]
- Bellomi M, Veronesi G, Trifirò G, et al. Computed tomography-guided preoperative radiotracer localization of nonpalpable lung nodules. Ann Thorac Surg 2010;90:1759-64. [Crossref] [PubMed]
- Ambrogi MC, Melfi F, Zirafa C, et al. Radio-guided thoracoscopic surgery (RGTS) of small pulmonary nodules. Surg Endosc 2012;26:914-9. [Crossref] [PubMed]
- Galetta D, Bellomi M, Grana C, et al. Radio-Guided Localization and Resection of Small or Ill-Defined Pulmonary Lesions. Ann Thorac Surg 2015;100:1175-80. [Crossref] [PubMed]
- Sortini D, Feo CV, Carcoforo P, et al. Thoracoscopic localization techniques for patients with solitary pulmonary nodule and history of malignancy. Ann Thorac Surg 2005;79:258-62; discussion 262. [Crossref] [PubMed]
- Abbas A, Kadakia S, Ambur V, et al. Intraoperative electromagnetic navigational bronchoscopic localization of small, deep, or subsolid pulmonary nodules. J Thorac Cardiovasc Surg 2017;153:1581-90. [Crossref] [PubMed]
- Anayama T, Hirohashi K, Miyazaki R, et al. Near-infrared dye marking for thoracoscopic resection of small-sized pulmonary nodules: comparison of percutaneous and bronchoscopic injection techniques. J Cardiothorac Surg 2018;13:5. [Crossref] [PubMed]
- Rho J, Lee JW, Quan YH, et al. Fluorescent and Iodized Emulsion for Preoperative Localization of Pulmonary Nodules. Ann Surg 2021;273:989-96. [PubMed]
- Li X, Xu K, Cen R, et al. Preoperative computer tomography-guided indocyanine green injection is associated with successful localization of small pulmonary nodules. Transl Lung Cancer Res 2021;10:2229-36. [Crossref] [PubMed]
- Okusanya OT, Holt D, Heitjan D, et al. Intraoperative near-infrared imaging can identify pulmonary nodules. Ann Thorac Surg 2014;98:1223-30. [Crossref] [PubMed]
- Quan YH, Oh CH, Jung D, et al. Evaluation of Intraoperative Near-Infrared Fluorescence Visualization of the Lung Tumor Margin With Indocyanine Green Inhalation. JAMA Surg 2020;155:732-40. [PubMed]
- Wang K, Huang W, Chen X, et al. Efficacy of Near-Infrared Fluorescence Video-Assisted Thoracoscopic Surgery for Small Pulmonary Nodule Resection with Indocyanine Green Inhalation: A Randomized Clinical Trial. Ann Surg Oncol 2023;30:5912-22. [Crossref] [PubMed]
- Zhang RR, Schroeder AB, Grudzinski JJ, et al. Beyond the margins: real-time detection of cancer using targeted fluorophores. Nat Rev Clin Oncol 2017;14:347-64. [Crossref] [PubMed]
- Schouw HM, Huisman LA, Janssen YF, et al. Targeted optical fluorescence imaging: a meta-narrative review and future perspectives. Eur J Nucl Med Mol Imaging 2021;48:4272-92. [Crossref] [PubMed]
- Sarkaria IS, Martin LW, Rice DC, et al. Pafolacianine for intraoperative molecular imaging of cancer in the lung: The ELUCIDATE trial. J Thorac Cardiovasc Surg 2023;166:e468-78. [Crossref] [PubMed]
- Bhatt KM, Tandon YK, Graham R, et al. Electromagnetic Navigational Bronchoscopy versus CT-guided Percutaneous Sampling of Peripheral Indeterminate Pulmonary Nodules: A Cohort Study. Radiology 2018;286:1052-61. [Crossref] [PubMed]
- DiBardino DM, Yarmus LB, Semaan RW. Transthoracic needle biopsy of the lung. J Thorac Dis 2015;7:S304-16. [PubMed]
- Heerink WJ, de Bock GH, de Jonge GJ, et al. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol 2017;27:138-48. [Crossref] [PubMed]
- Huo YR, Chan MV, Habib AR, et al. Pneumothorax rates in CT-Guided lung biopsies: a comprehensive systematic review and meta-analysis of risk factors. Br J Radiol 2020;93:20190866. [Crossref] [PubMed]
- Larscheid RC, Thorpe PE, Scott WJ. Percutaneous transthoracic needle aspiration biopsy: a comprehensive review of its current role in the diagnosis and treatment of lung tumors. Chest 1998;114:704-9. [Crossref] [PubMed]
- Li H, Boiselle PM, Shepard JO, et al. Diagnostic accuracy and safety of CT-guided percutaneous needle aspiration biopsy of the lung: comparison of small and large pulmonary nodules. AJR Am J Roentgenol 1996;167:105-9. [Crossref] [PubMed]
- Fielding DIK, Bashirzadeh F, Son JH, et al. First Human Use of a New Robotic-Assisted Fiber Optic Sensing Navigation System for Small Peripheral Pulmonary Nodules. Respiration 2019;98:142-50. [Crossref] [PubMed]
- Benn BS, Romero AO, Lum M, et al. Robotic-Assisted Navigation Bronchoscopy as a Paradigm Shift in Peripheral Lung Access. Lung 2021;199:177-86. [Crossref] [PubMed]
- Kalchiem-Dekel O, Connolly JG, Lin IH, et al. Shape-Sensing Robotic-Assisted Bronchoscopy in the Diagnosis of Pulmonary Parenchymal Lesions. Chest 2022;161:572-82. [Crossref] [PubMed]
- Styrvoky K, Schwalk A, Pham D, et al. Shape-Sensing Robotic-Assisted Bronchoscopy with Concurrent use of Radial Endobronchial Ultrasound and Cone Beam Computed Tomography in the Evaluation of Pulmonary Lesions. Lung 2022;200:755-61. [Crossref] [PubMed]
- Low SW, Lentz RJ, Chen H, et al. Shape-Sensing Robotic-Assisted Bronchoscopy vs Digital Tomosynthesis-Corrected Electromagnetic Navigation Bronchoscopy: A Comparative Cohort Study of Diagnostic Performance. Chest 2023;163:977-84. [Crossref] [PubMed]
- Aboudara M, Roller L, Rickman O, et al. Improved diagnostic yield for lung nodules with digital tomosynthesis-corrected navigational bronchoscopy: Initial experience with a novel adjunct. Respirology 2020;25:206-13. [Crossref] [PubMed]
- Avasarala SK, Roller L, Katsis J, et al. Sight Unseen: Diagnostic Yield and Safety Outcomes of a Novel Multimodality Navigation Bronchoscopy Platform with Real-Time Target Acquisition. Respiration 2022;101:166-73. [Crossref] [PubMed]
- Dunn BK, Blaj M, Stahl J, et al. Evaluation of Electromagnetic Navigational Bronchoscopy Using Tomosynthesis-Assisted Visualization, Intraprocedural Positional Correction and Continuous Guidance for Evaluation of Peripheral Pulmonary Nodules. J Bronchology Interv Pulmonol 2023;30:16-23. [Crossref] [PubMed]
- Gmehlin CG, Kurman JS, Benn BS. Size and vision: Impact of fluoroscopic navigation, digital tomosynthesis, and continuous catheter tip tracking on diagnostic yield of small, bronchus sign negative lung nodules. Respir Med 2022;202:106941. [Crossref] [PubMed]
- Du J, Fu YF, Lv YN. Preoperative localization for lung nodules: a meta-analysis of bronchoscopic versus computed tomography guidance. Wideochir Inne Tech Maloinwazyjne 2022;17:601-10. [Crossref] [PubMed]
- Bolton WD, Cochran T, Ben-Or S, et al. Electromagnetic Navigational Bronchoscopy Reduces the Time Required for Localization and Resection of Lung Nodules. Innovations (Phila) 2017;12:333-7. [Crossref] [PubMed]
- Tamura M, Oda M, Fujimori H, et al. New indication for preoperative marking of small peripheral pulmonary nodules in thoracoscopic surgery. Interact Cardiovasc Thorac Surg 2010;11:590-3. [Crossref] [PubMed]
- Nakata M, Saeki H, Yokoyama N, et al. Pulmonary function after lobectomy: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 2000;70:938-41. [Crossref] [PubMed]
- Nomori H, Ohtsuka T, Horio H, et al. Difference in the impairment of vital capacity and 6-minute walking after a lobectomy performed by thoracoscopic surgery, an anterior limited thoracotomy, an anteroaxillary thoracotomy, and a posterolateral thoracotomy. Surg Today 2003;33:7-12. [Crossref] [PubMed]
- Doo KW, Yong HS, Kim HK, et al. Needlescopic resection of small and superficial pulmonary nodule after computed tomographic fluoroscopy-guided dual localization with radiotracer and hookwire. Ann Surg Oncol 2015;22:331-7. [Crossref] [PubMed]
- Kang DY, Kim HK, Kim YK, et al. Needlescopy-assisted resection of pulmonary nodule after dual localisation. Eur Respir J 2011;37:13-7. [Crossref] [PubMed]
- Li A, Chan S, Thung KH. Pre-operative CT localization for patients with subsolid opacities expecting video-assisted thoracoscopic surgery-single center experience of fluorescent iodized emulsion and hook-wire localization technique. Br J Radiol 2020;93:20190938. [Crossref] [PubMed]
- Bawaadam H, Benn BS, Colwell EM, et al. Lung Nodule Marking With ICG Dye-Soaked Coil Facilitates Localization and Delayed Surgical Resection. Ann Thorac Surg Short Rep 2023;1:221-5. [Crossref]
Cite this article as: Marakini AB, Satterfield M, Stockdale GG, Benn BS. Localization approaches to improve surgical resection of peripheral pulmonary lesions: a clinical practice review. Curr Chall Thorac Surg 2024;6:12.


