
Bronchial stump coverage and reinforcement by adjacent tissue to prevent bronchopleural fistula after anatomical lung resection in high-risk patients: a narrative review
Introduction
Bronchopleural fistula (BPF) is one of the postoperative complications associated with increased surgical mortality following lung resection. The overall incidence of BPF after pulmonary resection, including various types of surgery, has been reported as 0.30–2.1%, of which more recent studies show lower incidences (1-4). On the other hand, considering the recent advances in the perioperative treatment of lung cancer, there is a concern that the incidence of delayed BPF may rise again.
In this article, we review the recent management of BPF, highlighting the clinical risks and surgical and non-surgical management. We especially focus on the current evidence behind the coverage of bronchial stumps to prevent or reduce the mortality of BPF. We present this article in accordance with the Narrative Review reporting checklist (available at https://ccts.amegroups.com/article/view/10.21037/ccts-25-2/rc).
Methods
A literature search was performed in December 2024 using the PubMed database. Broad search terms included “bronchial stump” and “coverage” or “reinforcement”. Papers published in peer-reviewed journals between 1990 and 2024 were included. Case reports and articles published in languages other than English were excluded. One hundred and one articles were flagged with this initial screening of the literature. We did not consider the underlying disease for the lung resection, and therefore, lung resection for malignancy and infections were both included in this review. We further inspected the citations used in these articles to complement the literature search. Thus, in total, 46 were used in this review (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | 31st December, 2024 |
| Database searched | PubMed |
| Search terms used | “Bronchial stump” AND (“coverage” OR “reinforcement”) |
| Timeframe | 1990–2024 |
| Exclusion criteria | Case reports and articles published in languages other than English were excluded |
| Selection process | Conducted by T.K. |
Incidence and mortality rate of BPF after lung resection
The incidence of BPF after lobectomy has been reported as 0.44–1.2% (1,4-6). Pneumonectomy has been reported to develop a higher incidence of BPF, up to 3–10.8% (1,2,4,6-11). BPF incidence after pneumonectomy is high, possibly resulting from anatomical factors, whilst BPF after lobectomy is significantly less frequent and may arise due to technical errors or impaired healing caused by pre-existing medical conditions (12). Mortality rate after BPF has been reported as 14–71%, with recent studies showing better survival rates (1,3,13-16). According to the French database, the mortality rate of BPF was 25.9% for lobectomy, 16.7% for bilobectomy, and 20% for pneumonectomy (6).
Respiratory failure due to aspiration of infected pleural fluid through the fistula and sudden death by massive bleeding due to bronchovascular fistula are known as the major causes of death related to BPF (1,15,17). Hollaus et al. reported that 64/96 (67%) of the patients who developed BPF after pneumonectomy died during the observation period, and the leading cause of death was aspiration pneumonia in 25 patients (39%) (13).
Risks of BPF development
BPF risks after any lung resection
To date, various retrospective studies and meta-analyses have explored the risks of BPF development after lung resection. Right lower lobectomy, right middle and lower bilobectomy, and right pneumonectomy are associated with an increased risk of BPF compared with other types of lung resection (4,14). Male sex, pre-operative radiation, residual disease at the bronchial stump, and a history of diabetes mellitus, systemic steroids, chronic obstructive pulmonary disease, previous tuberculosis, older age, presence of pneumonia, and acute exacerbation of interstitial pneumonia have also been reported as clinical risk factors for BPF (1,7,9,14,18-24).
BPF risks related to lobectomy
Ichinose et al. retrospectively reviewed 3,180 patients who underwent lobectomy and reported that all 14 patients who developed BPF were men and had undergone right lower lobectomy. Multivariable analysis in the subgroup of men who underwent right lower lobectomy revealed that a high level of pre-operative serum C-reactive protein and a history of gastric cancer surgery were significantly associated with BPF (3).
BPF risks related to bronchoplasty
Peng et al. retrospectively reviewed 503 patients who underwent bronchoplasty and reported that preoperative Charlson Comorbidity Index (CCI) ≥2, right middle and/or lower lobectomy, and residual tumor in the bronchial margin as independent risk factors of BPF (15).
BPF risks related to preoperative respiratory infection
The presence of infection is also considered a risk of BPF. Panagopoulos et al. reported that 5/221 (2.3%) patients developed BPF after pneumonectomies for non-small cell lung cancer (NSCLC), and preoperative respiratory infection remained an independent risk factor of BPF in multivariate analysis (25). The reported incidence of BPF after pneumonectomy or lobectomy in patients with multidrug-resistant tuberculosis was 9/56 (16.1%) (26). Mitchell et al. reported that the incidence of postoperative BPF after anatomical lung resection in patients with nontuberculous mycobacterial diseases was 11/265 (4.2%), including 9/27 (33%) patients who underwent right pneumonectomy (27).
BPF risks related to the method used for stump closure
Whether the use of a stapler or manual suture is associated with BPF risk has been investigated in several studies. In a review article involving eight studies published in 2014, Zakkar et al. concluded there was no evidence that manual closure can decrease the risk of BPF (28). In 2022, Skrzypczak et al. also reported that the method used for stump closure did not impact BPF risk in their retrospective analysis of 455 post-pneumonectomy patients (10).
BPF risks related to neoadjuvant treatment
In the recent meta-analysis of 30 studies involving 14,912 patients who underwent lung cancer resection, Li et al. reported that neoadjuvant radiotherapy alone or neoadjuvant chemoradiotherapy were significantly associated with an increased risk of BPF but not neoadjuvant chemotherapy alone (29). The risk of BPF after neoadjuvant immunochemotherapy remains to be investigated. Of note, Zhao et al. recently reported four original cases of BPF after neoadjuvant immunochemotherapy from their institution and an additional four cases from other studies (30). They concluded that the central type lung cancer with stage III may be the risk factor of BPF in cases of neoadjuvant immunochemotherapy, although this needs further investigation.
Treatment for BPF after lung resection
The principal management of BPF is to control the infection and close the fistula. Chest tube drainage and intravenous antibiotics should be initiated as soon as possible. Open window thoracostomy, i.e., a surgical procedure that creates an opening in the chest wall by removing ribs and intercostal muscles allowing for ongoing drainage of the pleural cavity, should be considered for pleural empyema with BPF to enhance drainage and avoid aspiration pneumonia (8,14). If the onset of BPF is early (<14 days after surgery) and infection can be controlled, primary closure can be offered. Bronchoscopic closure of the fistula can be considered if the fistula is small (<5 mm), with local application of silver nitrate (31), fibrin glues (32), or autologous fat tissue (33). Application of airway occlusion stenting or Amplatzer plug (used for interventional cardiology) has also been recently reported as an alternative method in bronchoscopic closure of the fistula (34-36). Secondary closure of the fistula and obliteration of the residual infected space can be accomplished using the transposition of muscular flaps of the latissimus dorsi muscle, serratus anterior muscle, or pectoralis major muscles. An omental flap is also applied for plombage (8,14).
Bronchial stump covering and reinforcement by adjacent tissue
To minimize the fistula and separate the bronchial stump and adjacent vessels, various tissues, including pedicled intercostal muscle, pericardium, thymus, or pericardial fat, are used to cover the bronchial stump after lung resection (1,3,11,37).
Mounting evidence suggests that bronchial stump coverage also effectively reduces the risk of BPF (Table 2). To date, one randomized prospective trial compared the rate of BPF between the groups of patients with and without bronchial stump coverage by pedicled intercostal muscle flap (38). In this study, all patients had a history of diabetes mellitus and underwent pneumonectomy for lung cancer. Bronchial stump coverage (n=33) had a lower incidence of BPF development (0% vs. 8.8%, P=0.02) than no coverage (n=35). Other retrospective studies have shown conflicting results. However, interpreting these findings requires caution. Since bronchial stump coverage is typically used for high-risk patients, the observed association between BPF incidence and bronchial stump coverage may be biased. Indeed, various studies have reported no significant association between bronchial stump coverage and reduced rate of BPF (10,19,25,41-45), or even a significant association between bronchial stump coverage and increased incidence of BPF (18,46-48). Nevertheless, several retrospective studies support the association between bronchial stump coverage and the reduced rate of postoperative BPF (Table 2). Wang et al. showed that bronchial stump coverage was significantly associated with a lower incidence of BPF after pneumonectomy or lobectomy for 56 multidrug-resistant tuberculosis patients (26). In the retrospective analysis of 511 patients who underwent pneumonectomy for lung cancer, Mammana et al. showed that bronchial stump coverage significantly reduced the risk of postoperative BPF (39). In the retrospective analysis of 3,180 patients who underwent lobectomy for lung cancer, Ichinose et al. reported that 14 (0.44%) developed BPF. These were all male patients who underwent right lower lobectomy. Therefore, they further conducted multivariable regression analysis in the subgroup of male patients who underwent right lower lobectomy, and they showed that bronchial stump coverage was significantly associated with reduced risk of BPF (3).
Table 2
| Authors [year] (ref.) | Study design | Adjustment of confounders | Number | Cohort characteristics | Type of surgery | Tissue used for BSC† | Results |
|---|---|---|---|---|---|---|---|
| Sfyridis et al. [2007] (38) | Prospective | Randomization | 70 | Lung cancer patients with diabetes mellitus | Pneumonectomy | Intercostal muscle flap | BSC (n=33) had a lower incidence of BPF development (0% vs. 8.8%, P=0.02) than no BSC (n=35) |
| Wang et al. [2008] (26) | Retrospective | Multivariable regression | 56 | Multidrug-resistant tuberculosis | Pneumonectomy (n=25), lobectomy (n=31) | Parietal pleura (n=30), pericardium (n=12) | BSC (n=42) was inversely related to the rate of BPF (OR =0.061; P<0.05) |
| Mammana et al. [2020] (39) | Retrospective | Multivariable regression | 511 | Lung cancer patients | Pneumonectomy | pericardium (n=331), intercostal muscle (n=13), serratus anterior muscle (n=4), parietal pleura (n=11) | No BSC (n=152) was associated with increased risk of BPF (OR =5.2; 95% CI: 2.00–13.52; P=0.0007) |
| Ceylan et al. [2022] (40) | Retrospective | N/A | 187 | Lung cancer patients | Pneumonectomy | Thymopericardial fat flap | BSC (n=53) had a lower incidence of BPF development (1.9% vs. 11.2%, P=0.044) than no BSC (n=134) |
| Ichinose et al. [2023] (3) | Retrospective | Multivariable regression in subgroup | 330‡ | Lung cancer patients (subgroup analysis restricted to male patients with right lower lobectomy) | Lobectomy | Intercostal muscle flap (open thoracotomy), free pericardial fat pad (VATS) | BSC (n=195) was inversely associated with the development of BPF (OR =0.06; 95% CI: 0.00–0.77; P=0.031) |
†, pedicled tissue flap was used unless specified as a non-pedicled free graft (e.g., free pericardial fat pad). ‡, the full cohort was 3,180 patients who underwent lobectomy. BSC, bronchial stump coverage; BPF, bronchopleural fistula; CI, confidence interval; N/A, not applicable; OR, odds ratio; VATS, video-assisted thoracoscopic surgery.
Characteristics of tissues used for bronchial stump coverage
Ongoing debates exist regarding the indication of bronchial stump coverage and the optimal tissue choice. Skrzypczak et al. reported that bronchial stump covering with the parietal pleura, intercostal muscle flap, or pericardial fat pad (5.88%, 9.09%, and 6.36%, respectively; P=0.88) had no significant influence on the frequency of BPF after pneumonectomy for lung cancer (10). On the other hand, the incidence of BPF after pneumonectomy for drug-resistant cavitary tuberculosis was higher when free pericardial fat was used compared with a pedicled intercostal muscle flap (49).
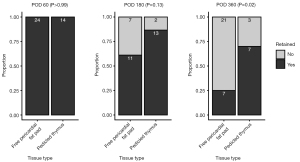
Pedicled muscle flaps, including intercostal muscle and latissimus dorsi muscle, can be easily harvested under open thoracotomy, whilst free pericardial fat pad and pedicled thymus can be harvested under a minimally invasive approach. To deepen our understanding of these tissue grafts, we retrospectively analyzed the retention rate of the engrafted tissue after bronchial stump coverage under video-assisted thoracoscopic surgery (VATS) using postoperative computed tomography (CT) scans (50,51). The criteria for bronchial stump coverage in our institution included but were not limited to, a history of poorly controlled diabetes mellitus, systemic steroid therapy, lung infection, or prior mediastinal radiotherapy. The retention rate at postoperative day (POD) 60 was 100% in both free pericardial fat pad and pedicled thymus, and the difference in retention rate at POD 180 did not reach statistical significance (pedicled thymus, 86.7%; free pericardial fat, 61.1%; P=0.13); however, the long-term retention rate at POD 360 of pedicled thymus was significantly higher than that of free pedicled fat pad (pedicled thymus, 70.0%; free pericardial fat, 25.0%; P=0.02) (Figure 1). Previous studies have shown that the median time from surgery to BPF is 20–30 days after lobectomy, whilst a longer and wider range of intervals has been reported after pneumonectomy (3,4,8,11,31). A free pericardial fat pad may be sufficient for most high-risk cases undergoing lobectomy but may not be sufficient for patients with the risk of delayed BPF. A practically important advantage of a free pericardial fat pad may be that, empirically, harvesting the free pericardial fat graft is less complicated and less time-consuming than a pedicled thymic flap. We concluded that the type of graft used to cover the bronchial stump should be carefully selected based on the risk, feasibility, anatomy, and surgical approach in each case.
Limitations of the review and need future research
Although we have adequately reviewed the published literature on bronchial stump coverage after lung resection, there is only one randomized prospective trial investigating the efficacy of bronchial stump coverage, which showed a significantly decreased risk of BPF by applying an intercostal muscle flap. Retrospective studies have provided controversial results, influenced by the high-risk nature of patients receiving bronchial stump coverage. However, because the covering technique is feasible under both open thoracotomy and minimally invasive approaches, it may be difficult to conduct a randomized study in terms of ethical concerns to compare bronchial stump coverage and no coverage for high-risk patients considering the morbidity and mortality of BPF once it happens. Besides, various tissues are applied for bronchial stump coverage, and the efficacy of each method remains unclear. Again, it may not be feasible to conduct a randomized study to compare across various methods, considering the low frequency of BPF. In addition, the risk of BPF after neoadjuvant immunotherapy has become a recent topic in postoperative BPF management. Accumulation of large-scale and high-quality clinical data that allows adequate control of risk factors and confounders, including neoadjuvant immunotherapy, may be needed for future studies.
Conclusions
Although rare, BPF treatment and prevention remain a major challenge in thoracic surgery. Postoperative BPF is associated with high mortality, and it often requires highly invasive treatment, including open-wound thoracotomy. Recent advances in perioperative treatment may increase the incidence of BPF after lung cancer resection. To reduce surgical morbidity and mortality and to improve patient care, it is essential to revisit the management of postoperative BPF and understand the characteristics of each bronchial stump coverage technique. Bronchial stump coverage, especially using grafts that can be harvested under a minimally invasive approach, may as well be proactively applied for patients with a risk of BPF.
Acknowledgments
None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://ccts.amegroups.com/article/view/10.21037/ccts-25-2/rc
Peer Review File: Available at https://ccts.amegroups.com/article/view/10.21037/ccts-25-2/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts-25-2/coif). T.K. was supported by the JSPS Overseas Research Fellowships Program (No. 202060447) from April 2020 to January 2023, which was unrelated to the present manuscript. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Asamura H, Naruke T, Tsuchiya R, et al. Bronchopleural fistulas associated with lung cancer operations. Univariate and multivariate analysis of risk factors, management, and outcome. J Thorac Cardiovasc Surg 1992;104:1456-64.
- Wang Y, Zhu M, Pan Y, et al. Long-term follow up and comparison between conservative and interventional therapy in postoperative bronchopleural fistula-a cohort study. J Thorac Dis 2023;15:1210-6. [Crossref] [PubMed]
- Ichinose J, Hashimoto K, Matsuura Y, et al. Risk factors for bronchopleural fistula after lobectomy for lung cancer. J Thorac Dis 2023;15:3330-8. [Crossref] [PubMed]
- Matsunaga T, Suzuki K, Hattori A, et al. Risk factors for bronchopleural fistula based on surgical procedure and sex in 4794 consecutive patients undergoing anatomical pulmonary resection. Surg Today 2024;54:617-26. [Crossref] [PubMed]
- Matsuoka K, Imanishi N, Yamada T, et al. Clinical results of bronchial stump coverage using free pericardial fat pad. Interact Cardiovasc Thorac Surg 2016;23:553-9. [Crossref] [PubMed]
- Pforr A, Pagès PB, Baste JM, et al. A Predictive Score for Bronchopleural Fistula Established Using the French Database Epithor. Ann Thorac Surg 2016;101:287-93. [Crossref] [PubMed]
- Hu XF, Duan L, Jiang GN, et al. A clinical risk model for the evaluation of bronchopleural fistula in non-small cell lung cancer after pneumonectomy. Ann Thorac Surg 2013;96:419-24. [Crossref] [PubMed]
- Mazzella A, Pardolesi A, Maisonneuve P, et al. Bronchopleural Fistula After Pneumonectomy: Risk Factors and Management, Focusing on Open-Window Thoracostomy. Semin Thorac Cardiovasc Surg 2018;30:104-13. [Crossref] [PubMed]
- Gursoy S, Yazgan S, Ucvet A, et al. Postpneumonectomy bronchopleural fistula in non-small cell lung cancer patients: incidence, survival, mortality, and treatment analysis. Surg Today 2018;48:695-702. [Crossref] [PubMed]
- Skrzypczak P, Roszak M, Kasprzyk M, et al. The technique of stump closure has no impact on post-pneumonectomy bronchopleural fistula in the non-small cell lung cancer-a cross-sectional study. J Thorac Dis 2022;14:3343-51. [Crossref] [PubMed]
- Steimer D, Coughlin JM, Yates E, et al. Empiric flap coverage for the pneumonectomy stump: How protective is it? A single-institution cohort study. J Thorac Cardiovasc Surg 2024;167:849-58. [Crossref] [PubMed]
- Skrzypczak P, Kasprzyk M, Gabryel P, et al. Methods of bronchial stump buttressing in post-pneumonectomy bronchopleural fistula prevention: a systematic review. Pol Przegl Chir 2024;96:70-84. [Crossref] [PubMed]
- Hollaus PH, Lax F, el-Nashef BB, et al. Natural history of bronchopleural fistula after pneumonectomy: a review of 96 cases. Ann Thorac Surg 1997;63:1391-6; discussion 1396-7. [Crossref] [PubMed]
- Gritsiuta AY, Eguchi T, Jones DR, et al. A Stepwise Approach for Postlobectomy Bronchopleural Fistula. Oper Tech Thorac Cardiovasc Surg 2020;25:85-104. [Crossref] [PubMed]
- Peng Z, Mei J, Liu C, et al. Risk factors and outcomes of bronchopleural fistula after bronchoplasty in patients with non-small cell lung cancer: a retrospective multivariate analysis. Transl Lung Cancer Res 2022;11:744-56. [Crossref] [PubMed]
- Yang YH, Park SY, Kim HE, et al. Postoperative bronchopleural fistula repair: Surgical outcomes and adverse factors for its success. Thorac Cancer 2022;13:1401-5. [Crossref] [PubMed]
- Khargi K, Duurkens VA, Knaepen PJ, et al. Hemorrhage due to inflammatory erosion of the pulmonary artery stump in postpneumonectomy bronchopleural fistula. Ann Thorac Surg 1993;56:357-8. [Crossref] [PubMed]
- Tokunaga Y, Kita Y, Okamoto T. Analysis of Risk Factors for Bronchopleural Fistula after Surgical Treatment of Lung Cancer. Ann Thorac Cardiovasc Surg 2020;26:311-9. [Crossref] [PubMed]
- Okuda M, Go T, Yokomise H. Risk factor of bronchopleural fistula after general thoracic surgery: review article. Gen Thorac Cardiovasc Surg 2017;65:679-85. [Crossref] [PubMed]
- Jichen QV, Chen G, Jiang G, et al. Risk factor comparison and clinical analysis of early and late bronchopleural fistula after non-small cell lung cancer surgery. Ann Thorac Surg 2009;88:1589-93. [Crossref] [PubMed]
- d'Amato TA, Ashrafi AS, Schuchert MJ, et al. Risk of pneumonectomy after induction therapy for locally advanced non-small cell lung cancer. Ann Thorac Surg 2009;88:1079-85. [Crossref] [PubMed]
- Uramoto H, Hanagiri T. The development of bronchopleural fistula in lung cancer patients after major surgery: 31 years of experience with 19 cases. Anticancer Res 2011;31:619-24.
- Kobayashi S, Karube Y, Nishihira M, et al. Postoperative pyothorax a risk factor for acute exacerbation of idiopathic interstitial pneumonia following lung cancer resection. Gen Thorac Cardiovasc Surg 2016;64:476-80. [Crossref] [PubMed]
- Cerfolio RJ. The incidence, etiology, and prevention of postresectional bronchopleural fistula. Semin Thorac Cardiovasc Surg 2001;13:3-7. [Crossref] [PubMed]
- Panagopoulos ND, Apostolakis E, Koletsis E, et al. Low incidence of bronchopleural fistula after pneumonectomy for lung cancer. Interact Cardiovasc Thorac Surg 2009;9:571-5. [Crossref] [PubMed]
- Wang H, Lin H, Jiang G. Pulmonary resection in the treatment of multidrug-resistant tuberculosis: a retrospective study of 56 cases. Ann Thorac Surg 2008;86:1640-5. [Crossref] [PubMed]
- Mitchell JD, Bishop A, Cafaro A, et al. Anatomic lung resection for nontuberculous mycobacterial disease. Ann Thorac Surg 2008;85:1887-92; discussion 1892-3. [Crossref] [PubMed]
- Zakkar M, Kanagasabay R, Hunt I. No evidence that manual closure of the bronchial stump has a lower failure rate than mechanical stapler closure following anatomical lung resection. Interact Cardiovasc Thorac Surg 2014;18:488-93. [Crossref] [PubMed]
- Li S, Fan J, Liu J, et al. Neoadjuvant therapy and risk of bronchopleural fistula after lung cancer surgery: a systematic meta-analysis of 14 912 patients. Jpn J Clin Oncol 2016;46:534-46. [Crossref] [PubMed]
- Zhao R, Guan X, Zhang P, et al. Development of postoperative bronchopleural fistula after neoadjuvant immunochemotherapy in non-small cell lung cancer: case reports and review of the literature. J Cancer Res Clin Oncol 2024;150:175. [Crossref] [PubMed]
- Boudaya MS, Smadhi H, Zribi H, et al. Conservative management of postoperative bronchopleural fistulas. J Thorac Cardiovasc Surg 2013;146:575-9. [Crossref] [PubMed]
- Nagahiro I, Aoe M, Sano Y, et al. Bronchopleural fistula after lobectomy for lung cancer. Asian Cardiovasc Thorac Ann 2007;15:45-8. [Crossref] [PubMed]
- Bottoni E, Banzatti BP, Novellis P, et al. Endoscopic Lipofilling for the Treatment of Bronchopleural Fistulas After Anatomic Lung Resection. Ann Thorac Surg 2021;111:e143-5. [Crossref] [PubMed]
- Han X, Yin M, Li L, et al. Customized airway stenting for bronchopleural fistula after pulmonary resection by interventional technique: single-center study of 148 consecutive patients. Surg Endosc 2018;32:4116-24. [Crossref] [PubMed]
- Zeng J, Wu X, Chen Z, et al. Modified silicone stent for the treatment of post-surgical bronchopleural fistula: a clinical observation of 17 cases. BMC Pulm Med 2021;21:10. [Crossref] [PubMed]
- Menezes V, Soder S, Kadadah S, et al. Bronchoscopic treatment of a bronchopleural fistula after pneumonectomy. JTCVS Tech 2020;4:345-8. [Crossref] [PubMed]
- Klepetko W, Taghavi S, Pereszlenyi A, et al. Impact of different coverage techniques on incidence of postpneumonectomy stump fistula. Eur J Cardiothorac Surg 1999;15:758-63. [Crossref] [PubMed]
- Sfyridis PG, Kapetanakis EI, Baltayiannis NE, et al. Bronchial stump buttressing with an intercostal muscle flap in diabetic patients. Ann Thorac Surg 2007;84:967-71. [Crossref] [PubMed]
- Mammana M, Marulli G, Zuin A, et al. Postpneumonectomy bronchopleural fistula: analysis of risk factors and the role of bronchial stump coverage. Surg Today 2020;50:114-22. [Crossref] [PubMed]
- Ceylan KC, Batıhan G, Kaya ŞÖ. Novel method for bronchial stump coverage for prevents postpneumonectomy bronchopleural fistula: pedicled thymopericardial fat flap. J Cardiothorac Surg 2022;17:286. [Crossref] [PubMed]
- Sonobe M, Nakagawa M, Ichinose M, et al. Analysis of risk factors in bronchopleural fistula after pulmonary resection for primary lung cancer. Eur J Cardiothorac Surg 2000;18:519-23. [Crossref] [PubMed]
- Maniwa T, Saito Y, Kaneda H, et al. Bronchial stump reinforcement with the intercostal muscle flap without adverse effects. Eur J Cardiothorac Surg 2006;30:652-6. [Crossref] [PubMed]
- Gudbjartsson T, Gyllstedt E, Pikwer A, et al. Early surgical results after pneumonectomy for non-small cell lung cancer are not affected by preoperative radiotherapy and chemotherapy. Ann Thorac Surg 2008;86:376-82. [Crossref] [PubMed]
- Di Maio M, Perrone F, Deschamps C, et al. A meta-analysis of the impact of bronchial stump coverage on the risk of bronchopleural fistula after pneumonectomy. Eur J Cardiothorac Surg 2015;48:196-200. [Crossref] [PubMed]
- Caushi F, Qirjako G, Skenduli I, et al. Is the flap reinforcement of the bronchial stump really necessary to prevent bronchial fistula? J Cardiothorac Surg 2020;15:248. [Crossref] [PubMed]
- Algar FJ, Alvarez A, Aranda JL, et al. Prediction of early bronchopleural fistula after pneumonectomy: a multivariate analysis. Ann Thorac Surg 2001;72:1662-7. [Crossref] [PubMed]
- Deschamps C, Bernard A, Nichols FC 3rd, et al. Empyema and bronchopleural fistula after pneumonectomy: factors affecting incidence. Ann Thorac Surg 2001;72:243-7; discussion 248. [Crossref] [PubMed]
- Miller DL, Deschamps C, Jenkins GD, et al. Completion pneumonectomy: factors affecting operative mortality and cardiopulmonary morbidity. Ann Thorac Surg 2002;74:876-83; discussion 883-4. [Crossref] [PubMed]
- Bazhenov AV, Mariandyshev AO, Hinderaker SG, et al. Prevention of bronchial fistulas after pneumonectomies for selected cavitary drug resistant lung tuberculosis. Front Surg 2023;10:1151137. [Crossref] [PubMed]
- Karasaki T, Fujimori S, Suzuki S, et al. Retention Rate of Free Pericardial Fat Grafts after Bronchial Stump Coverage. Thorac Cardiovasc Surg 2024;72:646-50. [Crossref] [PubMed]
- Karasaki T, Fujimori S, Suzuki S, et al. Long-term retention of the pedicled thymic flap after bronchial stump coverage. Interdiscip Cardiovasc Thorac Surg 2025;40:ivaf012. [Crossref] [PubMed]
Cite this article as: Karasaki T, Mihara S, Fujimori S. Bronchial stump coverage and reinforcement by adjacent tissue to prevent bronchopleural fistula after anatomical lung resection in high-risk patients: a narrative review. Curr Chall Thorac Surg 2025;7:7.


