
Lung transplantation in the era of normothermic regional perfusion in donation after cardiac death: a review
Introduction
Background
Lung transplantation (LT) remains the only potentially curative option for many forms of end-stage pulmonary disease. In 2022, 2,743 LTs were conducted in the United States (1). While only 3.7% of adult LTs were from donors after cardiac death (DCD) in 2017, in 2022, 7.4% of cases were performed from DCDs (1). It is well established that after DCD, functional warm ischemia time and resultant hypoxia and hypercarbia can ensue, but the impact of this on the incidence of primary graft dysfunction (PGD) after LT is not well-elucidated (2). Unfortunately, the incidence of clinically significant (Grade III) PGD after LT is very high (reportedly as high as 30.8% at 72 hours post-LT) and is associated with increased mortality and long-term graft failure (3). Recent data from the International Society of Heart and Lung Transplantation (ISHLT) has demonstrated that 5-year survival after DCD-LT (9.5% of LTs between 2003–2017) was similar compared to donation after brain death (DBD)-LT (63% vs. 61%) (4).
Rationale and knowledge gap
Given the severe shortage of viable organs for transplant, there is hope that the use of normothermic regional perfusion (NRP) may help to alleviate the damage from warm ischemic times and potentially increase the viability of more marginal organs. NRP is a modality utilized during time of organ procurement in which the donor, after being formally pronounced deceased, is first placed on extracorporeal normothermic circulation in situ to maintain perfusion of transplantable organs and minimize warm ischemia injury.
While the use of ex vivo lung perfusion (EVLP) has increased the number of potentially viable, marginal donor organs, the implication of NRP for LT remains unclear and is undergoing extensive investigation (5). Indeed, between 2019–2022, only 14.9% of potentially available lungs after NRP in DCD patients were transplanted (6). NRP has been associated with excellent outcomes in other organ models including heart, kidney, and liver transplantation (7-9). The lungs are extremely susceptible to ischemic injury, and it is imperative that the community of transplant clinicians continue to explore opportunities to enhance viability of these precious organs, particularly with marginal donors. How donors are prepared for lung procurement on NRP, the technical principles of cannulation and restoration of perfusion after pronouncement of donor death, and opportunities to improve organ quality after procurement are major knowledge gaps.
Objective
In this review, we discuss this emerging and rapidly expanding role of NRP in the setting of DCD for LT. We review the major technical considerations of lung procurement in the setting of abdominal (A-NRP) or thoracoabdominal NRP (TA-NRP), reflect on early outcomes, and highlight the biologic and physiologic tenets of lung procurement with NRP. Finally, we discuss potential risks and opportunities to enhance allograft viability and summarize ethical considerations of LT with NRP.
Technical principles of NRP
NRP reflects an evolution in the management of a donor organ in situ to minimize the damage from ischemic injury prior to transplantation. As lungs are extremely susceptible to ischemia after procurement, NRP can be used to maximize perfusion of marginal DCD lungs. This may increase the quantity and quality of organs available for transplantation, addressing the disparity between organ supply and patient demand (10). Typically, the NRP process entails a 2–20-minute “no-touch” observation period after donor pulselessness is noted and the patient is assessed to be deceased (11). After this time, the organ procurement surgical team begins organ recovery. Extracorporeal membrane oxygenation (ECMO) cannulation is initiated first, and the patient is re-intubated (these often occur simultaneously). A nasogastric tube is also placed, if not already present, to reduce the risk of aspiration pneumonitis. During NRP, donor organs are perfused in situ prior to procurement as soon as the patient is pronounced formally deceased. With TA-NRP, this process involves median sternotomy, aortic and right atrial cannulation, occlusion of cerebral blood flow via clamping of the aortic arch vessels, and initiation of central venoarterial (VA)-ECMO/modified cardiopulmonary bypass (CPB) circuit which incorporates a reservoir (5,10). For A-NRP, ECMO is initiated in bifemoral fashion and typically utilized for isolated abdominal organ procurement. An important difference between the two regional perfusion modalities is that in A-NRP, the descending thoracic aorta is clamped [we suggest stapling the descending thoracic aorta in the chest as well as the inferior vena cava (IVC), superior vena cava (SVC), azygos vein, and arch vessels], which prevents perfusion cranial to the diaphragm; in TA-NRP, the thoracic aorta and its branches (excluding the aortic arch vessels) are perfused and the ascending aortic branch vessels are ligated (12). Other accepted techniques for vascular control include simple suture ligation, venting vessels to the NRP pump, or venting to air (12). Vascular clamping alone is not recommended due to the risk of accidental dislodgement. A-NRP inherently increases the risk of chest bleeding, which may increase ischemia risk for abdominal organs. Techniques described by Mohite et al. utilized to ameliorate the bleeding risk with A-NRP include early ligation of the azygous vein, clamping the IVC after initiation of NRP and placement of a pulmonary artery (PA) vent and opening the right and left atria for decompression prior to lung procurement (13). Regardless of NRP technique, meticulous dissection and careful use of electrocautery is crucial to minimize blood loss. NRP is generally continued for up to 60 minutes (12). Once sinus rhythm is obtained, the lungs and abdominal organs are perfused with pulsatile, physiologic pressure, which is an advantage of TA-NRP over A-NRP (Figure 1) (14).
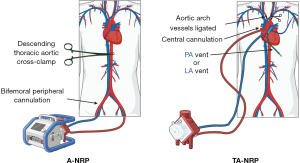
With respect to lung procurement with TA-NRP, protocols for organ retrieval are institution-specific. A well-established recently published protocol from the University of Colorado begins after the 5-minute no-touch interval with re-intubation, sternotomy, and bronchoscopic evaluation of the lung. Systemic anticoagulation is initiated 3 minutes prior to terminal extubation. Once the pericardium is opened, the brachiocephalic vessels are clamped. Intraoperative transesophageal echocardiography is frequently utilized to assess donor cardiac function on TA-NRP (15). Dual-stage venous cannulation through the right atrium is performed and the aortic cannula is advanced through a proximal arch aortotomy. NRP is initiated for 45–60 minutes. Typically, ventilator settings are adjusted to target a positive end-expiratory pressure of 5–10 mmHg, fraction of inspired oxygen is maintained at 40%, and lung-protective tidal volumes (4–8 cc/kg) are maintained (12). The lungs are explored bronchoscopically to assess tissue quality, clear mucus plugs, confirm pan-lobar aeration and to rule out anatomical variations. Blood gases are obtained to target an ideal PaO2/FiO2 (P/F) ratio >300 mmHg. Gross parenchymal evaluation includes assessment of any pathologic lesions, injuries, blebs, atelectasis, or edema (16). A main PA vent is also placed to empty the left heart, potentially reducing the degree of pulmonary edema associated with NRP. Some institutions utilize a left atrial (LA) appendage vent for similar decompressive effect. After the requisite NRP duration has passed, the donor is temporarily weaned off ECMO/CPB and then a formal assessment of the lungs is made. Pulmonary venous gases are drawn. The aorta is cross-clamped and the lungs are flushed with an extracellular dextran-based electrolyte solution with low potassium as well as with prostaglandin for lung preservation through the PA vent (15). Procured organs can either be implanted directly into the recipient or placed on EVLP (Figure 2).
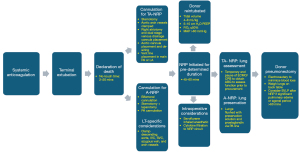
Equally important to NRP itself is careful concomitant cardiothoracic critical care to maximize lung viability. Inotropic and vasopressor support should target a goal mean arterial pressure of >60 mmHg (17). To maintain the integrity of the pulmonary allograft, diuretics should be administered prior to procurement to reduce the risk of pulmonary edema (12). The use of diuretics has to be carefully balanced with inotropic and vasopressor requirements to maintain end-organ perfusion prior to procurement. While on TA-NRP, Hoffman et al. recommend targeting a blood flow rate of >2.2 L/min/m2 (12). Serum hemoglobin is generally maintained at 8 mg/dL (18). As previously noted, low-tidal volume lung-protective strategies are routinely employed to minimize pulmonary vascular resistance elevation and to minimize atelectotrauma (15). Fraction of inspired oxygen and positive end-expiratory pressure are kept as physiologically low as possible, ideally at 40% and 5 mmHg, respectively (18).
Early clinical outcomes of DCD-NRP for LT
Currently, the use of DCD-NRP for LT is in its nascency with most centers reporting outcomes from a small volume of transplants. In these early studies, NRP for LT has been shown to be comparable in outcomes to direct procurement in DCD patients. A study of 8 DCD-LTs with NRP at a single institution demonstrated a 0% incidence of grade III PGD at 72 hours and no postoperative ECMO requirements. Furthermore, there were no significant differences in postoperative 24-hour P/F ratio, acute or long-term mortality at 1 year compared to the DBD control group (18). Tanaka et al. reported in a series of 28 LT patients that the use of A-NRP did not significantly impact the incidence of PGD, in-hospital mortality, or 1-year survival compared to standard rapid abdominal organ retrieval (19). Similarly, Mora et al. reported that the use of A-NRP in lung procurement after DCD in 60 patients between 2014–2021 at a single institution revealed no significant differences in 3-month or 5-year survival compared to DBD control patients (20). Interestingly, the A-NRP cohort did have a higher incidence of grade III PGD at 72 hours (10% vs. 3.4%), which did not impact survival. It is important to note that EVLP after NRP was not frequently utilized in either cohort (3.3% vs. 1.9%) (20). Regarding abdominal organ procurement, using TA-NRP has been shown to statistically increase the utilization rates for both liver and pancreas donation compared to DCD donors without the use of NRP (21). Additionally, NRP has been shown to improve graft survival and decrease rates of PGD in both liver and kidney transplants (9,21).
The use of EVLP after DCD-NRP continues to evolve; another recent preliminary study investigating lung utilization after EVLP with or without NRP demonstrated that the lung utilization rate was lower in the NRP cohort vs. the DCD only cohort (17% vs. 37%, respectively) but was not statistically significant (22). To this point, whether NRP followed by traditional cold storage vs. traditional harvest followed by EVLP vs. NRP followed by EVLP is the most effective strategy for minimizing ischemic injury to lung allografts is poorly elucidated. In the context of heart transplantation, a recent abstract demonstrated that NRP vs. ex vivo perfusion [TransMedics Organ Care System (OCS)] after DCD demonstrated no significant differences with respect to NRP vs. OCS (23). These mixed findings and actively ongoing investigations clearly suggest that further exploration into the use of NRP-DCD lungs with EVLP is warranted. From a financial standpoint, the cost of NRP is significantly lower than the costs associated with OCS. Using NRP typically costs an additional $4,000 per case, whereas the OCS console costs $250,000–300,000, and its single-use components cost $30,000–50,000 (14).
Biologic and physiologic impacts of NRP for LT
Biologically, NRP has several potential benefits for lung allografts. Ribeiro et al. demonstrated in a porcine model of TA-NRP during DCD lung procurement that lungs harvested using TA-NRP followed by EVLP had similar graft oxygenation, airway pressures, pulmonary compliance, and inflammatory cytokine levels compared to direct procurement followed by EVLP (24). Furthermore, there were no significant differences in histopathologic tissue injury scores between arms. PA pressure was higher at 3 hours (24). In preclinical renal and liver transplant models, NRP has been associated with superior allograft function with increased pro-angiogenic and immunoregulatory changes compared to no NRP (25,26). These immunoregulatory changes may be associated with suppressed initial inflammatory insult to the organ, which may reduce the risk of PGD. While such intensive immunologic analyses have not been performed for lung allografts on NRP, further investigation is certainly warranted the use of NRP for LT continues to evolve. To this point, biomarkers to predict PGD risk are under investigation, though not specifically in the context of NRP. These include serum cytokines and cellular injury markers like interleukin (IL)-8, receptor for advanced glycation end products (RAGE), and cell-free DNA in ex vivo perfusates (16,27,28). Further investigation into the impact of NRP on changes in biomarker expression and future PGD risk is necessary.
Potential risks with after NRP for LT after DCD and opportunities to enhance outcomes
It is important to note that there are some lung allograft-specific concerns with NRP; chief among them is the incidence of allograft pulmonary edema, which could potentially contribute to PGD (15). In preclinical porcine models, the degree of lung edema, as assayed by the degree of Steen loss (salt-based perfusate used during EVLP) and evidence of pulmonary edema on X-rays, was similar between direct procurement and TA-NRP (24). Furthermore, ECMO and CPB inherently induce a systemic inflammatory response with stimulation of the innate immune system (specifically the alternate complement activation cascade) and release of multiple pro-inflammatory and immunomodulatory cytokines including IL-1β, tumor necrosis factor-α (TNF-α), IL-6, IL-8, IL-10 (29,30). Sustained inflammatory response is directly associated with microvascular disruption, ischemia-reperfusion injury, and end-organ parenchymal injury (30,31). To this point, acquired von Willebrand disease, hyperfibrinolysis, and consumptive coagulopathy are well-established consequences of ECMO utilization (32). In neonates, prolonged ECMO utilization is associated with increased neutrophil phagocytotic capacity suggesting an acute inflammatory response (33). Following this corollary, it is plausible that a similar level of inflammatory response is expected after NRP, though the complexity of immune response in the setting of recent cardiac arrest and patient death and re-stimulation is profound and has not been fully investigated.
A plausible solution to the cytokine release from the NRP-ECMO circuit may be to utilize cytokine filters connected to the NRP-ECMO circuit. The data on cytokine adsorption therapy with mechanical circulatory support have been mixed, with worse outcomes in the setting of venovenous (VV)-ECMO for coronavirus disease 2019 (COVID-19) pneumonia, but potential benefit in CPB during urgent/emergent cardiac surgery including endocarditis (30,34). This therapy entails the use of a porous polymer filter to remove cytokines as plasma from the ECMO or CPB circuit (30). Cytokine adsorption has been introduced to reduce the inflammatory response after NRP-DCD. Baroni et al. reported 3 cases of the use of hemadsorption with NRP with kidney and liver procurement. They noted improvement in serum TNF-α levels at 120 minutes from the start of NRP and all transplanted organs functioned well (35). Certainly, further investigation with a larger patient sample is required to better characterize the impact of cytokine adsorption in these organ systems but also in LT, for which the role of cytokine adsorption is controversial and continues to evolve. A recent abstract by Vandendriessche et al. demonstrated that the use of cytokine adsorption did not significantly modulate inflammatory cytokine levels after TA-NRP after DCD in a porcine model (36). Conversely, Ehrsam et al. demonstrated in their porcine model specifically for LT that the use of hemadsorption during and after left LT (not specifically with NRP) significantly reduced serum IL-2, IL-1α, GM-CSF, and TNF-α levels. Furthermore, lungs treated with hemadsorption had lower peak airway pressures during treatment and after 24 hours, had improved oxygenation and decarboxylation compared to controls (31).
Another important lung protective strategy that is under investigation for DCD-LT that may readily apply to NRP includes the use of sevoflurane to minimize ischemia-reperfusion injury (37-39). Sevoflurane is an inhaled anesthetic that may ameliorate ischemia-reperfusion injury via anesthetic conditioning and has been shown to minimize injury from myocardial infarction (40). Anesthetic preconditioning with sevoflurane has even been shown in a randomized trial to reduce the incidence of late cardiac events after coronary artery bypass surgery (41). Bertani et al. demonstrated in a porcine model that inhaled sevoflurane in a preconditioned setting prior to left LT resulted in stable oxygen saturations, P/F ratios, and pH compared to controls. Furthermore, on histologic analysis, the extent of PGD-related changes was significantly lower in the sevoflurane cohort (40). Notably, there was reduced alveolar edema after implantation in the sevoflurane arm (40). This is particularly important, as pulmonary edema after NRP is a known complication; it is possible that adjuncts like preconditioning with inhaled sevoflurane may help to ameliorate this risk.
Ethical considerations
Despite its potential to enable in-situ organ evaluation and increase donor organ utilization, the use of NRP has raised ethical concerns that have impacted its widespread adoption. The “dead donor rule”, one of the ethical foundations of organ transplantation states that organ donors must be deceased before organ procurement begins, and the act of organ procurement must not cause the death of the donor (42). In the transplant community, there is concern that TA-NRP violates the dead donor rule. A major tenet of this argument is rooted in the definition of circulation. As DCD donors are declared deceased based on circulatory and respiratory criteria, several hold the belief that using NRP to restore circulation negates the declaration of death (43). Another argument is that even with occluded aortic arch vessels, perfusion of the thoracic cavity may also result in cerebral perfusion via possible collateral circulation from the internal mammary, epigastric, or spinal arteries (44). In theory, with enough collateral flow to the brain, some donor brain function could be restored, violating the dead donor rule and the principle of nonmaleficence (43). However, one study using intraoperative transcranial Doppler in two TA-NRP DCD donor cases showed no brain blood flow after initiation of TA-NRP with ligation of only the aortic arch vessels (45). Although TA-NRP may potentially increase the availability of viable thoracic organs for transplant, resuming cardiac perfusion and this possible collateral circulation to the brain in a deceased patient raises ethical and legal dilemma that are not present with A-NRP (44). Our institutional policy permits use of organs procured with NRP, but not the performance of NRP itself. To this point, as these challenges are addressed and the role of TA-NRP evolves in the context of DCD-LT, it is imperative that institutions develop clear protocols and guidelines to navigate the complex ethical landscape while maximizing the benefits of organ preservation and transplantation.
Strengths and limitations
This review provides a current and extensive analysis of the emerging clinical impact and roles of TA- and A-NRP in LT. It provides a detailed account of recently developed NRP techniques and perioperative critical care considerations, and effectively highlights the current data regarding short-term outcomes. However, this field is very much in its nascency, and available data and studies are primarily from non-randomized studies with small patient populations. Furthermore, the paucity of reported long-term outcomes of LT after NRP in DCD procurements restricts the ability to draw definitive consensus guidelines about its broader clinical impact or applicability. As more long-term data become available, further discussion of the risks, benefits, and ethical considerations will be important.
Conclusions
LT in the 21st century continues to evolve with ever-expanding opportunities to maximize the use of marginal organs, particularly in the setting of DCD. NRP is a promising strategy to facilitate in situ perfusion of these fragile organs that are extremely sensitive to hemodynamic shifts and warm ischemic times, particularly in the setting of DCD, which inherently increases the risk of marginal parenchyma. Early clinical outcomes of the use of NRP for DCD-LT are promising and largely equivalent to traditional procurement strategies. As organ procurement services continue to increasingly utilize NRP for enhancing organ viability, it will be crucial to continue to explore novel approaches to maximize utilization of marginal organs, standardize cannulation and perioperative critical care management of organs on perfusion, and to continue to discuss as a medical community the ethical ramifications of this decidedly complex intervention. This review is unique in its analysis of the evolving role of TA- and A-NRP in LT, synthesizing the latest evidence, clinical applications, and emerging challenges while offering novel insights into adjunctive strategies to optimize NRP outcomes. Further investigation into continuing to enhance outcomes after NRP and the role of NRP and EVLP together to maximize lung allograft viability for transplantation is warranted.
Acknowledgments
None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Haytham Elgharably) for the series “Lung Transplantation: New Frontiers” published in Current Challenges in Thoracic Surgery. The article has undergone external peer review.
Peer Review File: Available at https://ccts.amegroups.com/article/view/10.21037/ccts-24-42/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts-24-42/coif). The series “Lung Transplantation: New Frontiers” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Valapour M, Lehr CJ, Schladt DP, et al. OPTN/SRTR 2022 Annual Data Report: Lung. Am J Transplant 2024;24:S394-456. [Crossref] [PubMed]
- Avtaar Singh SS, Das De S, Al-Adhami A, et al. Primary graft dysfunction following lung transplantation: From pathogenesis to future frontiers. World J Transplant 2023;13:58-85. [Crossref] [PubMed]
- Diamond JM, Lee JC, Kawut SM, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med 2013;187:527-34. [Crossref] [PubMed]
- Van Raemdonck D, Keshavjee S, Levvey B, et al. 5-year results from the ISHLT DCD lung transplant registry confirm excellent recipient survival from donation after circulatory death donors. J Heart Lung Transplant 2019;38:S103. [Crossref]
- Keshavamurthy S, Rodgers-Fischl P. Donation after circulatory death (DCD)-lung procurement. Indian J Thorac Cardiovasc Surg 2021;37:425-32. [Crossref] [PubMed]
- Malas J, Chen Q, Thomas J, et al. The impact of thoracoabdominal normothermic regional perfusion on early outcomes in donation after circulatory death lung transplantation. J Heart Lung Transplant 2023;42:1040-4. [Crossref] [PubMed]
- Smith DE, Kon ZN, Carillo JA, et al. Early experience with donation after circulatory death heart transplantation using normothermic regional perfusion in the United States. J Thorac Cardiovasc Surg 2022;164:557-568.e1. [Crossref] [PubMed]
- Brubaker AL, Sellers MT, Abt PL, et al. US Liver Transplant Outcomes After Normothermic Regional Perfusion vs Standard Super Rapid Recovery. JAMA Surg 2024;159:677-85. [Crossref] [PubMed]
- Padilla M, Coll E, Fernández-Pérez C, et al. Improved short-term outcomes of kidney transplants in controlled donation after the circulatory determination of death with the use of normothermic regional perfusion. Am J Transplant 2021;21:3618-28. [Crossref] [PubMed]
- Truog RD, Doernberg SN. In Defense of Normothermic Regional Perfusion. Hastings Cent Rep 2024;54:24-31. [Crossref] [PubMed]
- Gardiner D, McGee A. Death, permanence and current practice in donation after circulatory death. QJM 2017;110:199-201. [PubMed]
- Hoffman JRH, Hartwig MG, Cain MT, et al. Consensus Statement: Technical Standards for Thoracoabdominal Normothermic Regional Perfusion. Ann Thorac Surg 2024;118:778-91. [Crossref] [PubMed]
- Mohite PN, Messer S, Curry P. Technique of blood conservation in direct perfusion and procurement of lungs in combination with abdominal normothermic regional perfusion. JHLT Open 2024;4:100087. [Crossref] [PubMed]
- Alamouti-Fard E, Garg P, Wadiwala IJ, et al. Normothermic Regional Perfusion is an Emerging Cost-Effective Alternative in Donation After Circulatory Death (DCD) in Heart Transplantation. Cureus 2022;14:e26437. [Crossref] [PubMed]
- Cain MT, Park SY, Schäfer M, et al. Lung recovery utilizing thoracoabdominal normothermic regional perfusion during donation after circulatory death: The Colorado experience. JTCVS Tech 2023;22:350-8. [Crossref] [PubMed]
- Kukreja J, Campo-Canaveral de la Cruz JL, Van Raemdonck D, et al. The 2024 American Association for Thoracic Surgery expert consensus document: Current standards in donor lung procurement and preservation. J Thorac Cardiovasc Surg 2025;169:484-504. [Crossref] [PubMed]
- Fiedler AG, DeVries S, Czekajlo C, et al. Normothermic regional perfusion surgical technique for the procurement of cardiac donors after circulatory death. JTCVS Tech 2022;12:113-5. [Crossref] [PubMed]
- Chang SH, Geraci TC, Piper GL, et al. Outcomes of lung and heart-lung transplants utilizing donor after circulatory death with thoracoabdominal normothermic regional perfusion. JHLT Open 2024;4:100058. [Crossref] [PubMed]
- Tanaka S, Luis Campo-Cañaveral de la Cruz J, Crowley Carrasco S, et al. Effect on the donor lungs of using abdominal normothermic regional perfusion in controlled donation after circulatory death. Eur J Cardiothorac Surg 2020;ezaa398. [PubMed]
- Mora V, Ballesteros MA, Naranjo S, et al. Lung transplantation from controlled donation after circulatory death using simultaneous abdominal normothermic regional perfusion: A single center experience. Am J Transplant 2022;22:1852-60. [Crossref] [PubMed]
- Bekki Y, Croome KP, Myers B, et al. Normothermic Regional Perfusion Can Improve Both Utilization and Outcomes in DCD Liver, Kidney, and Pancreas Transplantation. Transplant Direct 2023;9:e1450. [Crossref] [PubMed]
- Francois SA, Popa S, Shaver CM, et al. (1234) Comparison of lung utilization from NRP-DCD vs non-NRP DCD using EVLP. J Heart Lung Transplant 2023;42:S527. [Crossref]
- Bashian E, Gardner G, Ambrosio M, et al. Outcomes of DCD Heart Transplant: NRP and OCS. J Heart Lung Transplant 2024;43:S31. [Crossref]
- Ribeiro RVP, Reynolds FA, Sarrafian TL, et al. Impact of normothermic regional perfusion during DCD recovery on lung allograft function: A preclinical study. JHLT Open 2023;2:100009. [Crossref] [PubMed]
- Kerforne T, Allain G, Giraud S, et al. Defining the optimal duration for normothermic regional perfusion in the kidney donor: A porcine preclinical study. Am J Transplant 2019;19:737-51. [Crossref] [PubMed]
- Clatworthy MR, Watson CJE. Understanding the Immunology of Normothermic Machine Perfusion. Transpl Int 2023;36:11670. [Crossref] [PubMed]
- Hamilton BC, Kukreja J, Ware LB, et al. Protein biomarkers associated with primary graft dysfunction following lung transplantation. Am J Physiol Lung Cell Mol Physiol 2017;312:L531-41. [Crossref] [PubMed]
- Kanou T, Nakahira K, Choi AM, et al. Cell-free DNA in human ex vivo lung perfusate as a potential biomarker to predict the risk of primary graft dysfunction in lung transplantation. J Thorac Cardiovasc Surg 2021;162:490-499.e2. [Crossref] [PubMed]
- Millar JE, Fanning JP, McDonald CI, et al. The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology. Crit Care 2016;20:387. [Crossref] [PubMed]
- Naruka V, Salmasi MY, Arjomandi Rad A, et al. Use of Cytokine Filters During Cardiopulmonary Bypass: Systematic Review and Meta-Analysis. Heart Lung Circ 2022;31:1493-503. [Crossref] [PubMed]
- Ehrsam JP, Arni S, Weisskopf M, et al. Extracorporeal cytokine adsorption reduces systemic cytokine storm and improves graft function in lung transplantation. JTCVS Open 2023;15:497-507. [Crossref] [PubMed]
- Haus M, Foltan M, Philipp A, et al. Neutrophil extracellular traps - a potential trigger for the development of thrombocytopenia during extracorporeal membrane oxygenation. Front Immunol 2024;15:1339235. [Crossref] [PubMed]
- DePuydt LE, Schuit KE, Smith SD. Effect of extracorporeal membrane oxygenation on neutrophil function in neonates. Crit Care Med 1993;21:1324-7. [Crossref] [PubMed]
- Supady A, Weber E, Rieder M, et al. Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation (CYCOV): a single centre, open-label, randomised, controlled trial. Lancet Respir Med 2021;9:755-62. [Crossref] [PubMed]
- Baroni S, Melegari G, Brugioni L, et al. First experiences of hemoadsorption in donation after circulatory death. Clin Transplant 2020;34:e13874. [Crossref] [PubMed]
- Vandendriessche K, Brouckaert J, van Suylen V, et al. (871) Cytokine Profiles During Thoraco-Abdominal Normothermic Regional Perfusion (TA-NRP) in a Porcine Model. J Heart Lung Transplant 2023;42:S378. [Crossref]
- Yamada Y, Laube I, Jang JH, et al. Sevoflurane preconditioning protects from posttransplant injury in mouse lung transplantation. J Surg Res 2017;214:270-7. [Crossref] [PubMed]
- Wang X, Parapanov R, Francioli C, et al. Experimental ex vivo lung perfusion with sevoflurane: effects on damaged donor lung grafts. Interact Cardiovasc Thorac Surg 2018;26:977-84. [Crossref] [PubMed]
- Erquicia I, Cusati G, Del Barrio M, et al. Sevoflurane administration improves gas exchange in a lung autotransplant model: 5AP4–8. Eur J Anaesthesiol 2010;27:101. [Crossref]
- Bertani A, Miceli V, De Monte L, et al. Donor Preconditioning with Inhaled Sevoflurane Mitigates the Effects of Ischemia-Reperfusion Injury in a Swine Model of Lung Transplantation. Biomed Res Int 2021;2021:6625955. [Crossref] [PubMed]
- Garcia C, Julier K, Bestmann L, et al. Preconditioning with sevoflurane decreases PECAM-1 expression and improves one-year cardiovascular outcome in coronary artery bypass graft surgery. Br J Anaesth 2005;94:159-65. [Crossref] [PubMed]
- Robertson JA. The dead donor rule. Hastings Cent Rep 1999;29:6-14. [Crossref] [PubMed]
- Wall A, Testa G. The ethics surrounding normothermic regional perfusion in donors following circulatory death. Clin Liver Dis (Hoboken) 2024;23:e0193. [Crossref] [PubMed]
- Basmaji J, Weijer C, Skaro A, et al. Paving the Road for the Adoption of Normothermic Regional Perfusion in Canada. Crit Care Explor 2021;3:e0553. [Crossref] [PubMed]
- Frontera JA, Lewis A, James L, et al. Thoracoabdominal normothermic regional perfusion in donation after circulatory death does not restore brain blood flow. J Heart Lung Transplant 2023;42:1161-5. [Crossref] [PubMed]
Cite this article as: Chandra R, Hauptmann E, Keshavamurthy S. Lung transplantation in the era of normothermic regional perfusion in donation after cardiac death: a review. Curr Chall Thorac Surg 2025;7:9.


